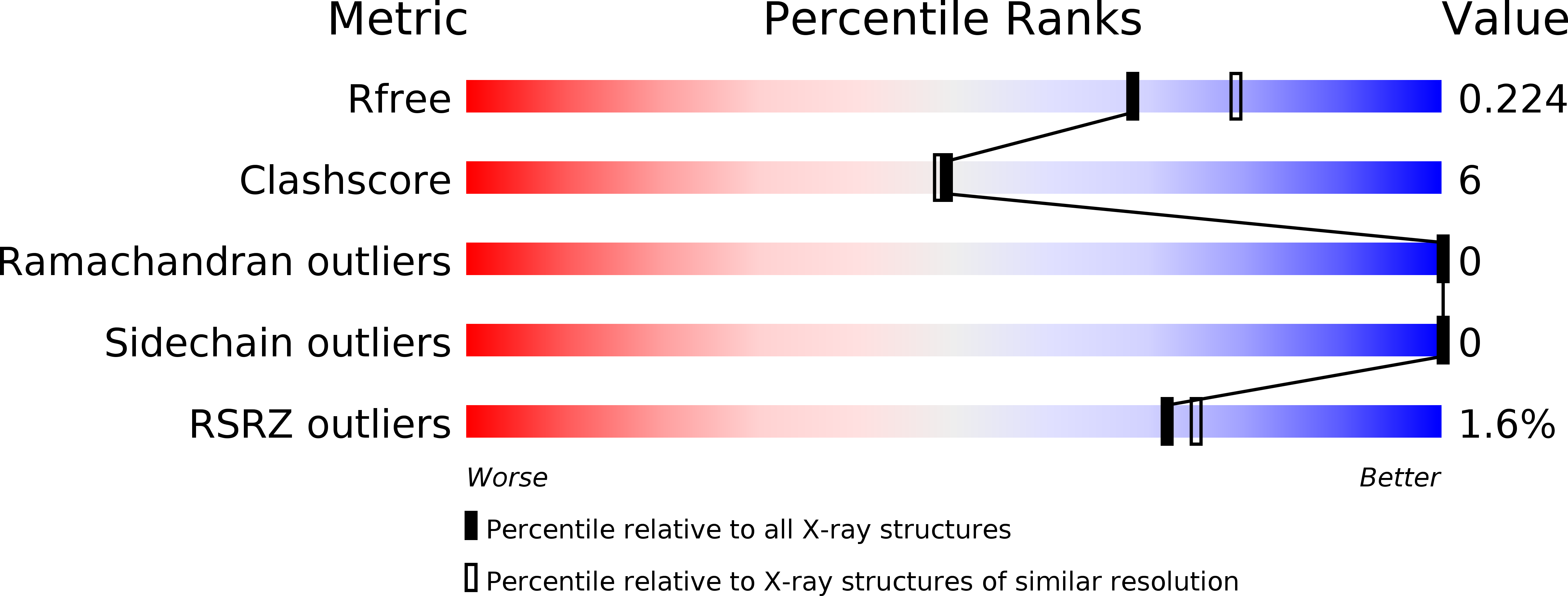
Polyhedra Structures and the Evolution of the Insect Viruses.
Ji, X., Axford, D., Owen, R., Evans, G., Ginn, H.M., Sutton, G., Stuart, D.I.(2015) J Struct Biol 192: 88
- PubMed: 26291392
- DOI: https://doi.org/10.1016/j.jsb.2015.08.009
- Primary Citation of Related Structures:
5A8S, 5A8T, 5A8U, 5A8V, 5A96, 5A98, 5A99, 5A9A, 5A9B, 5A9C, 5A9P - PubMed Abstract:
Polyhedra represent an ancient system used by a number of insect viruses to protect virions during long periods of environmental exposure. We present high resolution crystal structures of polyhedra for seven previously uncharacterised types of cypoviruses, four using ab initio selenomethionine phasing (two of these required over 100 selenomethionine crystals each). Approximately 80% of residues are structurally equivalent between all polyhedrins (pairwise rmsd ⩽ 1.5 Å), whilst pairwise sequence identities, based on structural alignment, are as little as 12%. These structures illustrate the effect of 400 million years of evolution on a system where the crystal lattice is the functionally conserved feature in the face of massive sequence variability. The conservation of crystal contacts is maintained across most of the molecular surface, except for a dispensable virus recognition domain. By spreading the contacts over so much of the protein surface the lattice remains robust in the face of many individual changes. Overall these unusual structural constraints seem to have skewed the molecule's evolution so that surface residues are almost as conserved as the internal residues.
Organizational Affiliation:
Division of Structural Biology, The Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford, Oxfordshire OX3 7BN, United Kingdom.