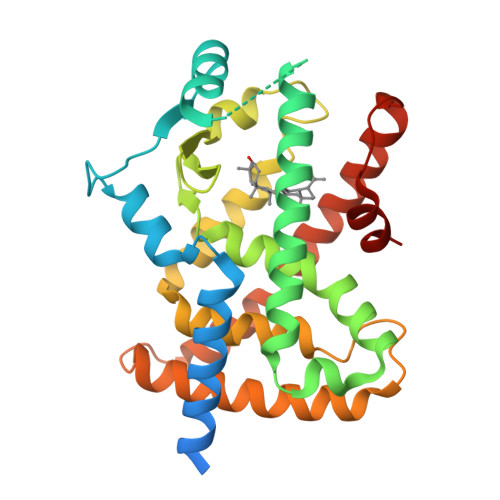
Betulinic acid is a PPAR gamma antagonist that improves glucose uptake, promotes osteogenesis and inhibits adipogenesis.
Brusotti, G., Montanari, R., Capelli, D., Cattaneo, G., Laghezza, A., Tortorella, P., Loiodice, F., Peiretti, F., Bonardo, B., Paiardini, A., Calleri, E., Pochetti, G.(2017) Sci Rep 7: 5777-5777
- PubMed: 28720829
- DOI: https://doi.org/10.1038/s41598-017-05666-6
- Primary Citation of Related Structures:
5LSG - PubMed Abstract:
PPAR antagonists are ligands that bind their receptor with high affinity without transactivation activity. Recently, they have been demonstrated to maintain insulin-sensitizing and antidiabetic properties, and they serve as an alternative treatment for metabolic diseases. In this work, an affinity-based bioassay was found to be effective for selecting PPAR ligands from the dried extract of an African plant (Diospyros bipindensis). Among the ligands, we identified betulinic acid (BA), a compound already known for its anti-inflammatory, anti-tumour and antidiabetic properties, as a PPARγ and PPARα antagonist. Cell differentiation assays showed that BA inhibits adipogenesis and promotes osteogenesis; either down-regulates or does not affect the expression of a series of adipogenic markers; and up-regulates the expression of osteogenic markers. Moreover, BA increases basal glucose uptake in 3T3-L1 adipocytes. The crystal structure of the complex of BA with PPARγ sheds light, at the molecular level, on the mechanism by which BA antagonizes PPARγ, and indicates a unique binding mode of this antagonist type. The results of this study show that the natural compound BA could be an interesting and safe candidate for the treatment of type 2 diabetes and bone diseases.
Organizational Affiliation:
Dipartimento di Scienze del Farmaco, Università degli Studi di Pavia, Via Taramelli 12, 27100, Pavia, Italy.