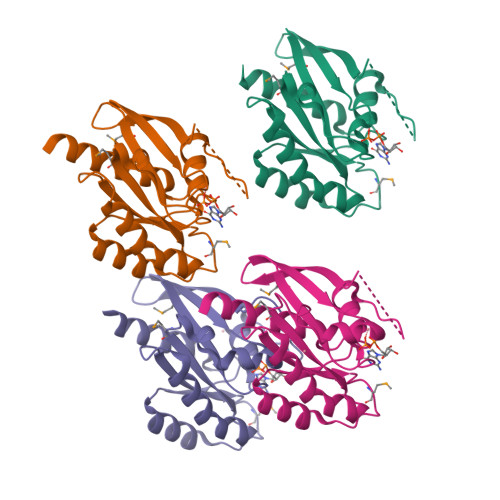
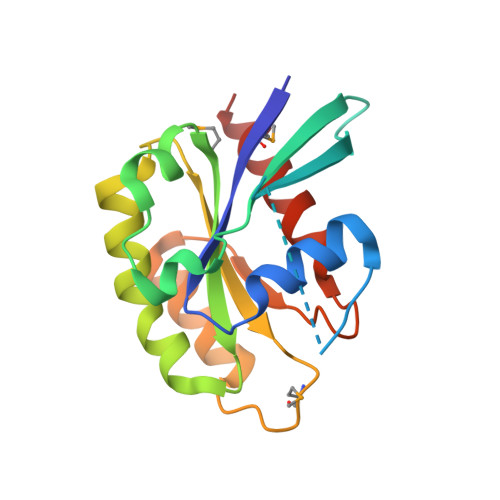
Crystal structures of a Rab protein in its inactive and active conformations.
Stroupe, C., Brunger, A.T.(2000) J Mol Biology 304: 585-598
- PubMed: 11099382
- DOI: https://doi.org/10.1006/jmbi.2000.4236
- Primary Citation of Related Structures:
1G16, 1G17 - PubMed Abstract:
We have determined crystal structures of Sec4, a member of the Rab family in the G protein superfamily, in two states: bound to GDP, and to a non-hydrolyzable GTP analog, guanosine-5'-(beta, gamma)-imidotriphosphate (GppNHp). This represents the first structure of a Rab protein bound to GDP. Sec4 in both states grossly resembles other G proteins bound to GDP and GppNHp. In Sec4-GppNHp, structural features common to active Rab proteins are observed. In Sec4-GDP, the switch I region is highly disordered and displaced relative to the switch I region of Ras-GDP. In two of the four molecules of Sec4-GDP in the asymmetric unit of the Sec4-GDP crystals, the switch II region adopts a conformation similar to that seen in the structure of the small G protein Ran bound to GDP. This allows residues threonine 76, glutamate 80, and arginine 81 of Sec4 to make contacts with other conserved residues and water molecules important for nucleotide binding. In the other two molecules in the asymmetric unit, these interactions do not take place. This structural variability in both the switch I and switch II regions of GDP-bound Sec4 provides a possible explanation for the high off-rate of GDP bound to Sec4, and suggests a mechanism for regulation of the GTPase cycle of Rab proteins by GDI proteins.
Organizational Affiliation:
The Howard Hughes Medical Institute and Departments of Molecular and Cellular Physiology, Stanford University, Stanford, CA, 94305-548, USA.