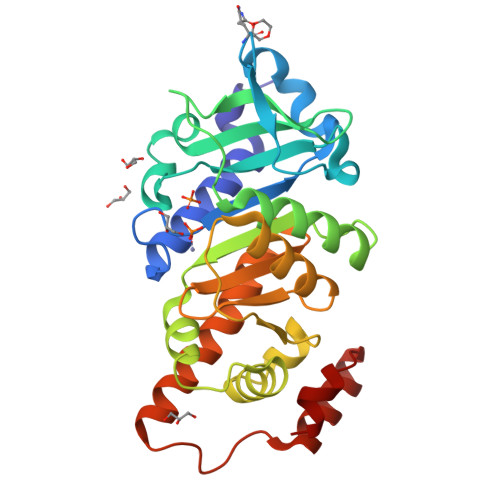
Structure and Catalytic Mechanism of L-Rhamnulose-1-Phosphate Aldolase.
Kroemer, M., Merkel, I., Schulz, G.E.(2003) Biochemistry 42: 10560
- PubMed: 12962479
- DOI: https://doi.org/10.1021/bi0349266
- Primary Citation of Related Structures:
1OJR - PubMed Abstract:
The structure of L-rhamnulose-1-phosphate aldolase has been established at 1.35 A resolution in a crystal form that was obtained by a surface mutation and has one subunit of the C(4)-symmetric tetramer in the asymmetric unit. It confirms an earlier 2.7 A resolution structure which was determined in a complicated crystal form with 20 subunits per asymmetric unit. The chain fold and the active center are similar to those of L-fuculose-1-phosphate aldolase and L-ribulose-5-phosphate 4-epimerase. The active center similarity is supported by a structural comparison of all three enzymes and by the binding mode of the inhibitor phosphoglycolohydroxamate at the site of the product dihydroxyacetone phosphate for the two aldolases. The sensitivity of the catalytic rate to several mutations and a comparison with the established mechanism of the related aldolase give rise to a putative catalytic mechanism. This mechanism involves the same binding mode of the second product L-lactaldehyde in both aldolases, except for a 180 degrees flip of the aldehyde group distinguishing between the two epimers rhamnulose and fuculose. The N-terminal domain exhibits a correlated anisotropic mobility that channels the isotropic Brownian motion into a directed movement of the catalytic base and the substrate phosphate on the N-domain toward the zinc ion and the lactaldehyde on the C-terminal domain. We suggest that this movement supports the catalysis mechanically.
Organizational Affiliation:
Institut für Organische Chemie und Biochemie, Albertstrasse 21, 79104 Freiburg im Breisgau, Germany.