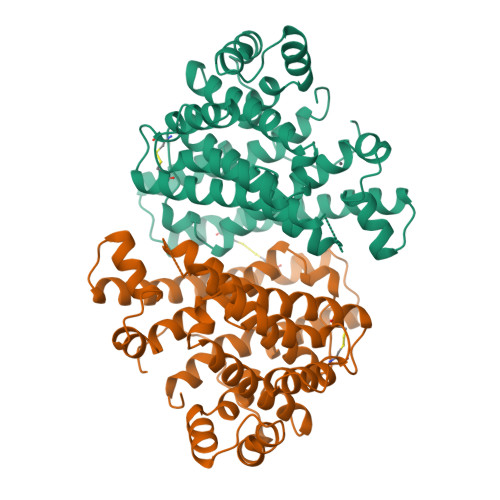
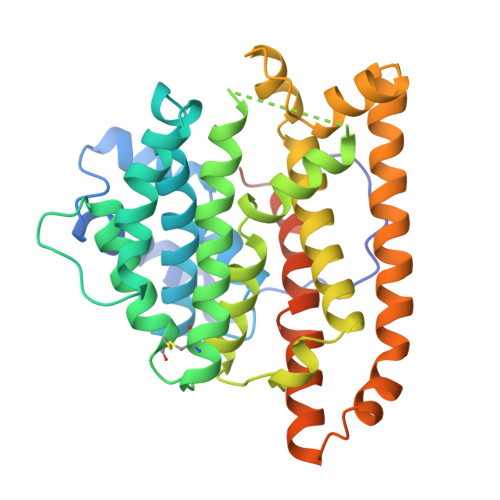
Crystal structure of pentalenene synthase: mechanistic insights on terpenoid cyclization reactions in biology.
Lesburg, C.A., Zhai, G., Cane, D.E., Christianson, D.W.(1997) Science 277: 1820-1824
- PubMed: 9295272
- DOI: https://doi.org/10.1126/science.277.5333.1820
- Primary Citation of Related Structures:
1PS1 - PubMed Abstract:
The crystal structure of pentalenene synthase at 2.6 angstrom resolution reveals critical active site features responsible for the cyclization of farnesyl diphosphate into the tricyclic hydrocarbon pentalenene. Metal-triggered substrate ionization initiates catalysis, and the alpha-barrel active site serves as a template to channel and stabilize the conformations of reactive carbocation intermediates through a complex cyclization cascade. The core active site structure of the enzyme may be preserved among the greater family of terpenoid synthases, possibly implying divergence from a common ancestral synthase to satisfy biological requirements for increasingly diverse natural products.
Organizational Affiliation:
Roy and Diana Vagelos Laboratories, Department of Chemistry, University of Pennsylvania, Philadelphia, PA 19104-6323, USA.