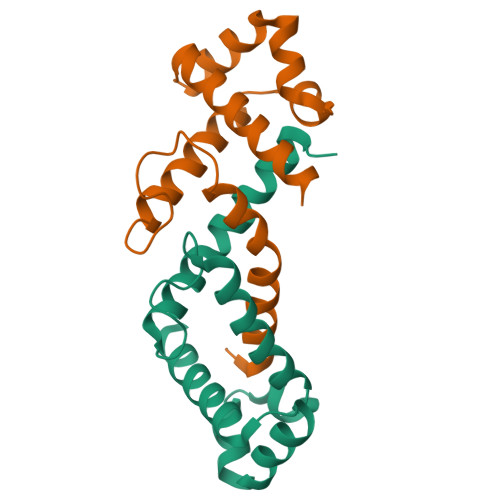
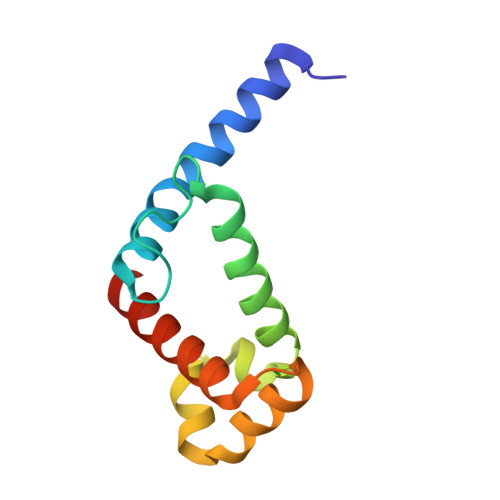
Crystal structure of the conserved subdomain of human protein SRP54M at 2.1 A resolution: evidence for the mechanism of signal peptide binding.
Clemons Jr., W.M., Gowda, K., Black, S.D., Zwieb, C., Ramakrishnan, V.(1999) J Mol Biology 292: 697-705
- PubMed: 10497032
- DOI: https://doi.org/10.1006/jmbi.1999.3090
- Primary Citation of Related Structures:
1QB2 - PubMed Abstract:
Protein SRP54 is an integral part of the mammalian signal recognition particle (SRP), a cytosolic ribonucleoprotein complex which associates with ribosomes and serves to recognize, bind, and transport proteins destined for the membrane or secretion. The methionine-rich M-domain of protein SRP54 (SRP54M) binds the SRP RNA and the signal peptide as the nascent protein emerges from the ribosome. A focal point of this critical cellular function is the detailed understanding of how different hydrophobic signal peptides are recognized efficiently and transported specifically, despite considerable variation in sequence. We have solved the crystal structure of a conserved functional subdomain of the human SRP54 protein (hSRP54m) at 2.1 A resolution showing a predominantly alpha helical protein with a large fraction of the structure available for binding. RNA binding is predicted to occur in the vicinity of helices 4 to 6. The N-terminal helix extends significantly from the core of the structure into a large but constricted hydrophobic groove of an adjacent molecule, thus revealing molecular details of possible interactions between alpha helical signal peptides and human SRP54.
Organizational Affiliation:
Department of Biochemistry, The University of Utah, School of Medicine, 50 North Medical Drive, Salt Lake City, UT 84132, USA.