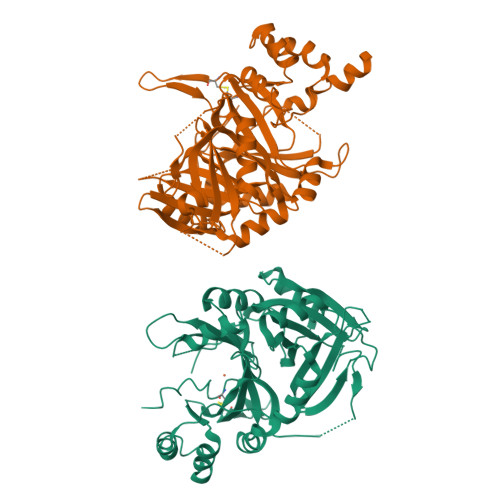
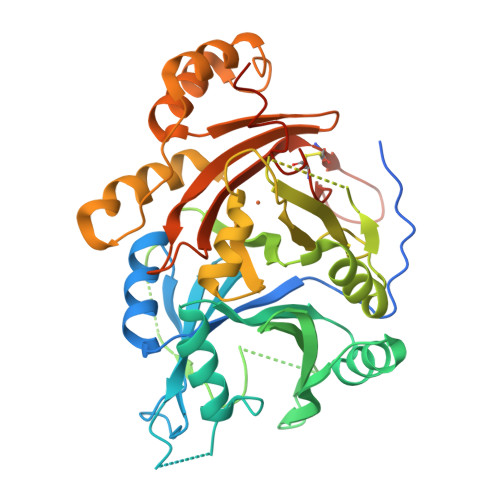
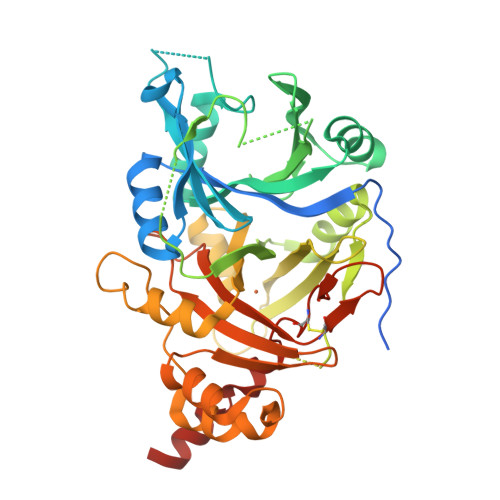
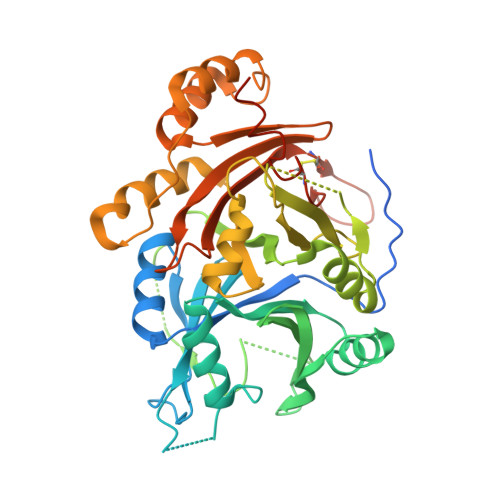
The crystal structures of Zea mays and Arabidopsis 4-Hydroxyphenylpyruvate Dioxygenase
Fritze, I.M., Linden, L., Freigang, J., Auerbach, G., Huber, R., Steinbacher, S.(2004) Plant Physiol 134: 1388-1400
- PubMed: 15084729
- DOI: https://doi.org/10.1104/pp.103.034082
- Primary Citation of Related Structures:
1SP8, 1SP9 - PubMed Abstract:
The transformation of 4-hydroxyphenylpyruvate to homogentisate, catalyzed by 4-hydroxyphenylpyruvate dioxygenase (HPPD), plays an important role in degrading aromatic amino acids. As the reaction product homogentisate serves as aromatic precursor for prenylquinone synthesis in plants, the enzyme is an interesting target for herbicides. In this study we report the first x-ray structures of the plant HPPDs of Zea mays and Arabidopsis in their substrate-free form at 2.0 A and 3.0 A resolution, respectively. Previous biochemical characterizations have demonstrated that eukaryotic enzymes behave as homodimers in contrast to prokaryotic HPPDs, which are homotetramers. Plant and bacterial enzymes share the overall fold but use orthogonal surfaces for oligomerization. In addition, comparison of both structures provides direct evidence that the C-terminal helix gates substrate access to the active site around a nonheme ferrous iron center. In the Z. mays HPPD structure this helix packs into the active site, sequestering it completely from the solvent. In contrast, in the Arabidopsis structure this helix tilted by about 60 degrees into the solvent and leaves the active site fully accessible. By elucidating the structure of plant HPPD enzymes we aim to provide a structural basis for the development of new herbicides.
Organizational Affiliation:
Max-Planck-Institut für Biochemie, Abteilung für Strukturforschung, 82152 Martinsried, Germany. fritze@biochem.mpg.de