Crystal Structure and Enzyme kinetics of the (S)-Specific 1-Phenylethanol Dehydrogenase of the denitrifying bacterium Strain EbN1
Hoeffken, H.W., Duong, M., Friedrich, T., Breuer, M., Hauer, B., Reinhardt, R., Rabus, R., Heider, J.(2006) Biochemistry 45: 82-93
- PubMed: 16388583
- DOI: https://doi.org/10.1021/bi051596b
- Primary Citation of Related Structures:
2EW8, 2EWM - PubMed Abstract:
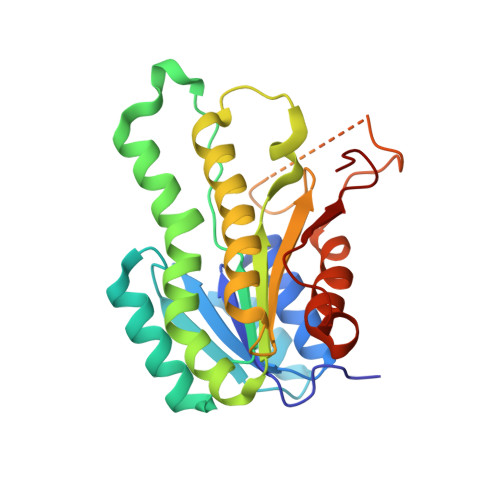
(S)-1-Phenylethanol dehydrogenase (PED) from the denitrifying bacterium strain EbN1 catalyzes the NAD+-dependent, stereospecific oxidation of (S)-1-phenylethanol to acetophenone and the biotechnologically interesting reverse reaction. This novel enzyme belongs to the short-chain alcohol dehydrogenase/aldehyde reductase family. The coding gene (ped) was heterologously expressed in Escherichia coli and the purified protein was crystallized. The X-ray structures of the apo-form and the NAD+-bound form were solved at a resolution of 2.1 and 2.4 A, respectively, revealing that the enzyme is a tetramer with two types of hydrophobic dimerization interfaces, similar to beta-oxoacyl-[acyl carrier protein] reductase (FabG) from E. coli. NAD+-binding is associated with a conformational shift of the substrate binding loop of PED from a crystallographically unordered "open" to a more ordered "closed" form. Modeling the substrate acetophenone into the active site revealed the structural prerequisites for the strong enantioselectivity of the enzyme and for the catalytic mechanism. Studies on the steady-state kinetics of PED indicated a highly positive cooperativity of both catalytic directions with respect to the substrates. This is contrasted by the behavior of FabG. Moreover, PED exhibits extensive regulation on the enzyme level, being inhibited by elevated concentrations of substrates and products, as well as the wrong enantiomer of 1-phenylethanol. These regulatory properties of PED are consistent with the presence of a putative "transmission module" between the subunits. This module consists of the C-terminal loops of all four subunits, which form a special interconnected structural domain and mediate close contact of the subunits, and of a phenylalanine residue in each subunit that reaches out between substrate-binding loop and C-terminal domain of an adjacent subunit. These elements may transmit the substrate-induced conformational change of the substrate binding loop from one subunit to the others in the tetrameric complex and thus mediate the cooperative behavior of PED.
Organizational Affiliation:
BASF AG, Physical Chemistry and Informatics, 67056 Ludwigshafen, Germany. wolfgang.hoeffken@basf-ag.de