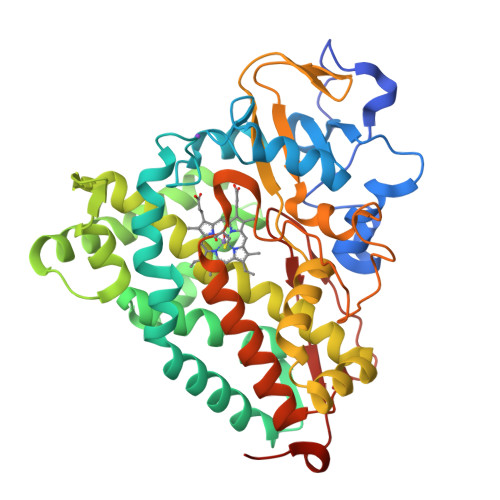
The status of high-valent metal oxo complexes in the P450 cytochromes.
Makris, T.M., Koenig, K., Schlichting, I., Sligar, S.G.(2006) J Inorg Biochem 100: 507-518
- PubMed: 16510191
- DOI: https://doi.org/10.1016/j.jinorgbio.2006.01.025
- Primary Citation of Related Structures:
2FE6, 2FER, 2FEU - PubMed Abstract:
The oxidative prowess of the P450 cytochromes in physiological reactions is attributed to the production of a high-valent iron-oxo complex, or Compound I intermediate, in the reaction cycle. Despite many years of study, however, the full electronic description of this fleeting intermediate still remains an active area of study. In this manuscript, the current status of the isolation and characterization of the P450 oxo-Fe(IV) is examined and compared to analogous states in related heme enzymes. In addition, the utilization of cofactor exchange to stabilize high-valent oxo-states in the P450 is addressed. Structural and spectroscopic studies on manganese reconstituted P450, and its corresponding oxo-complex, are presented.
Organizational Affiliation:
Department of Biochemistry, University of Illinois Urbana-Champaign, 116 Morrill Hall, Urbana, IL 61801, USA. tmakris@uiuc.edu