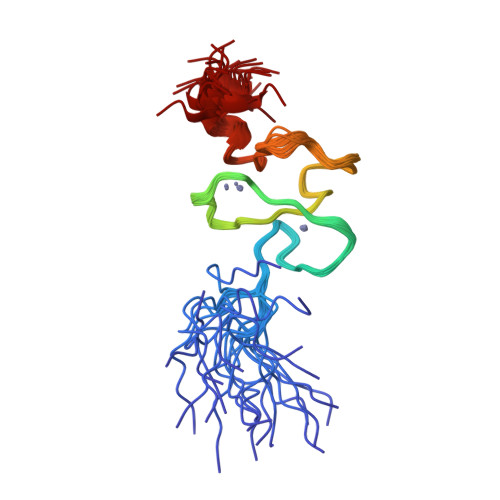
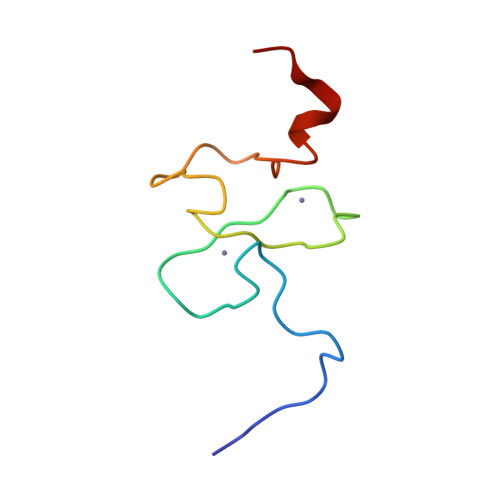
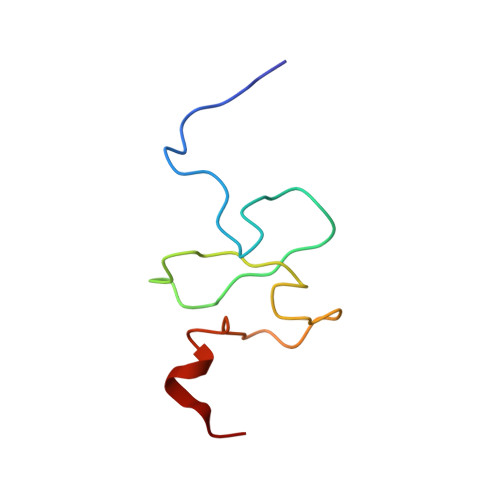
Functional impact of cancer-associated mutations in the tumor suppressor protein ING4.
Moreno, A., Palacios, A., Orgaz, J.L., Jimenez, B., Blanco, F.J., Palmero, I.(2010) Carcinogenesis 31: 1932-1938
- PubMed: 20705953
- DOI: https://doi.org/10.1093/carcin/bgq171
- Primary Citation of Related Structures:
2M1R - PubMed Abstract:
Inhibitor of growth 4 (ING4) is a member of the ING family of tumor suppressor proteins. In this study, we have analyzed the impact of two mutations in ING4 associated with human tumors (Y121N and N214D), testing their behavior in a series of functional, biochemical and structural analyses. We report that the N214D mutation dramatically dampened the ability of ING4 to inhibit proliferation, anchorage-independent growth or cell migration or to sensitize to cell death. In turn, the Y121N mutant did not differ significantly from wild-type ING4 in our assays. Neither of the mutations altered the normal subcellular localization of ING4, showing predominantly nuclear accumulation. We investigated the molecular basis of the defect in the activity of the N214D mutant. The folding and ability to bind histone marks of ING4 was not significantly altered by this mutation. Instead, we found that the functional impairment of the N214D mutant correlates with reduced protein stability due to increased proteasome-mediated degradation. In summary, our data demonstrates that a point mutation of ING4 associated to human tumors leads to the loss of several essential functions of ING4 pertinent to tumor protection and highlight the importance of ING4 function to prevent tumorigenesis.
Organizational Affiliation:
Instituto de Investigaciones Biomédicas Alberto Sols, Consejo Superior de Investigaciones Científicas-Universidad Autónoma de Madrid, Arturo Duperier 4, Madrid, Spain.