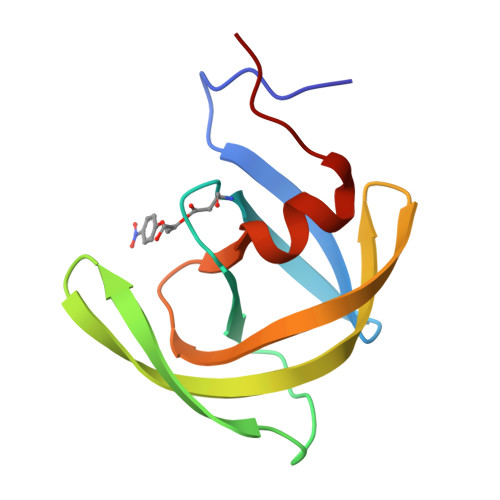
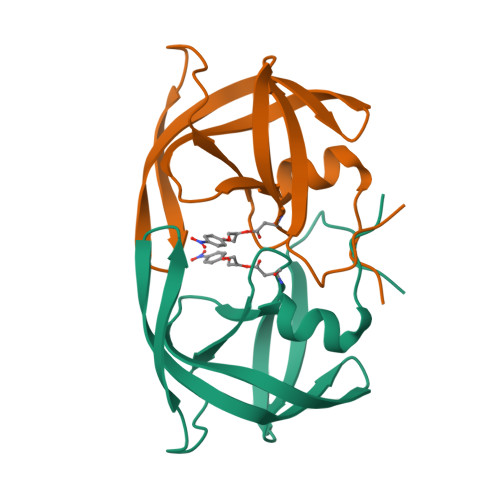
Structure of the protease from simian immunodeficiency virus: complex with an irreversible nonpeptide inhibitor.
Rose, R.B., Rose, J.R., Salto, R., Craik, C.S., Stroud, R.M.(1993) Biochemistry 32: 12498-12507
- PubMed: 8241141
- DOI: https://doi.org/10.1021/bi00097a030
- Primary Citation of Related Structures:
2SAM - PubMed Abstract:
A variant of the simian immunodeficiency virus protease (SIV PR), covalently bound to the inhibitor 1,2-epoxy-3-(p-nitrophenoxy)propane (EPNP), was crystallized. The structure of the inhibited complex was determined by X-ray crystallography to a resolution of 2.4 A and refined to an R factor of 19%. The variant, SIV PR S4H, was shown to diminish the rate of autolysis by at least 4-fold without affecting enzymatic parameters. The overall root mean square (rms) deviation of the alpha-carbons from the structure of HIV-1PR complexed with a peptidomimetic inhibitor (7HVP) was 1.16 A. The major differences are concentrated in three surface loops with rms differences between 1.2 and 2.1 A. For 60% of the molecule the rms deviation was only 0.6 A. The structure reveals one molecule of EPNP bound per protease dimer, a stoichiometry confirmed by mass spectral analysis. The epoxide moiety forms a covalent bond with either of the active site aspartic acids of the dimer, and the phenyl moiety occupies the P1 binding site. The EPNP nitro group interacts with Arg 8. This structure suggests a starting template for the design of nonpeptide-based irreversible inhibitors of the SIV and related HIV-1 and HIV-2 PRs.
Organizational Affiliation:
Department of Biochemistry, University of California at San Francisco 94143-0448.