Inhibition of the Glucosyltransferase Activity of Clostridial Rho/Ras-Glucosylating Toxins by Castanospermine.
Jank, T., Ziegler, M.O.P., Schulz, G.E., Aktories, K.(2008) FEBS Lett 582: 2277
- PubMed: 18505687
- DOI: https://doi.org/10.1016/j.febslet.2008.05.025
- Primary Citation of Related Structures:
2VL8 - PubMed Abstract:
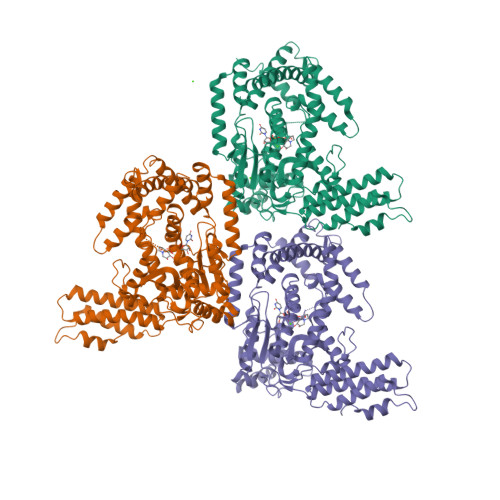
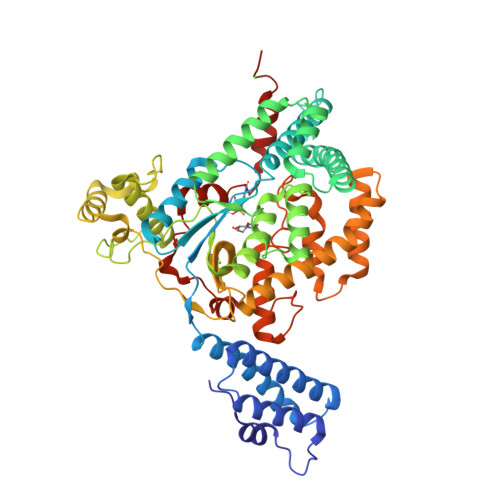
Castanospermine was identified as an inhibitor of the Rho/Ras-glucosylating Clostridium sordellii lethal toxin and Clostridium difficile toxin B. Microinjection of castanospermine into embryonic bovine lung cells prevented the cytotoxic effects of toxins. The crystal structure of the glucosyltransferase domain of C. sordellii lethal toxin in complex with castanospermine, UDP and a calcium ion was solved at a resolution of 2.3A. The inhibitor binds in a conformation that brings its four hydroxyl groups and its N-atom almost exactly in the positions of the four hydroxyls and of the ring oxygen of the glucosyl moiety of UDP-glucose, respectively.
Organizational Affiliation:
Institut für Experimentelle und Klinische Pharmakologie und Toxikologie, Albert-Ludwigs-Universität, Albertstrasse 25, Freiburg, Germany.