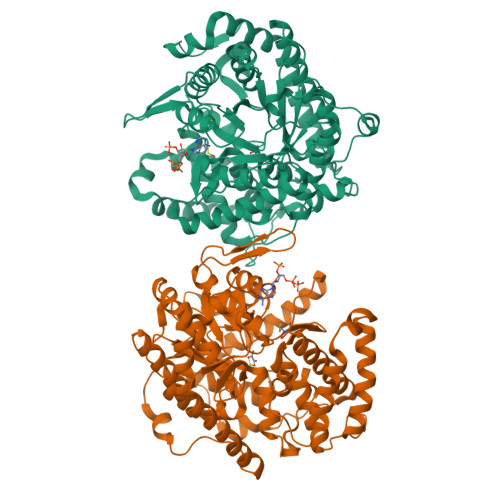
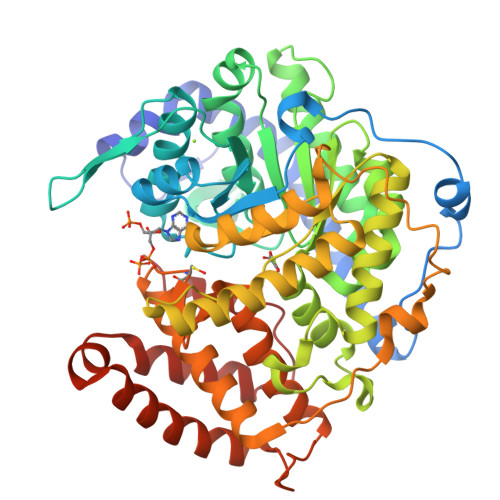
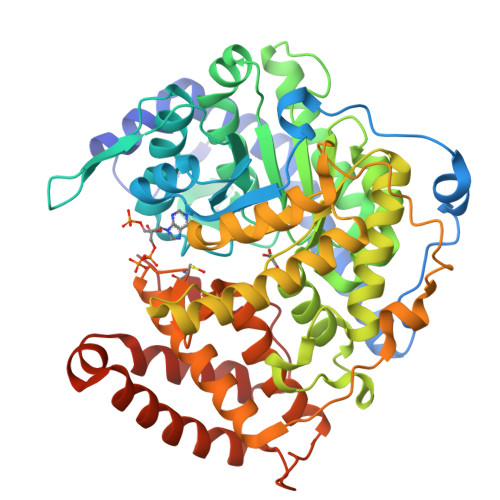
Atomic resolution structures of Escherichia coli and Bacillus anthracis malate synthase A: comparison with isoform G and implications for structure-based drug discovery
Lohman, J.R., Olson, A.C., Remington, S.J.(2008) Protein Sci 17: 1935-1945
- PubMed: 18714089
- DOI: https://doi.org/10.1110/ps.036269.108
- Primary Citation of Related Structures:
3CUX, 3CUZ, 3CV1, 3CV2 - PubMed Abstract:
Enzymes of the glyoxylate shunt are important for the virulence of pathogenic organisms such as Mycobacterium tuberculosis and Candida albicans. Two isoforms have been identified for malate synthase, the second enzyme in the pathway. Isoform A, found in fungi and plants, comprises approximately 530 residues, whereas isoform G, found only in bacteria, is larger by approximately 200 residues. Crystal structures of malate synthase isoform G from Escherichia coli and Mycobacterium tuberculosis were previously determined at moderate resolution. Here we describe crystal structures of E. coli malate synthase A (MSA) in the apo form (1.04 A resolution) and in complex with acetyl-coenzyme A and a competitive inhibitor, possibly pyruvate or oxalate (1.40 A resolution). In addition, a crystal structure for Bacillus anthracis MSA at 1.70 A resolution is reported. The increase in size between isoforms A and G can be attributed primarily to an inserted alpha/beta domain that may have regulatory function. Upon binding of inhibitor or substrate, several active site loops in MSA undergo large conformational changes. However, in the substrate bound form, the active sites of isoforms A and G from E. coli are nearly identical. Considering that inhibitors bind with very similar affinities to both isoforms, MSA is as an excellent platform for high-resolution structural studies and drug discovery efforts.
Organizational Affiliation:
Department of Physics, Institute of Molecular Biology, University of Oregon, Eugene, OR 97403-1229, USA.