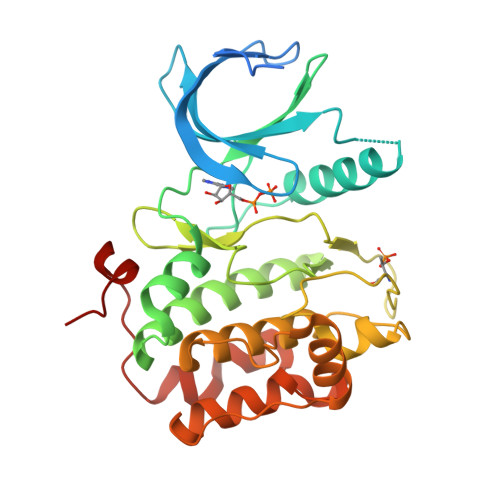
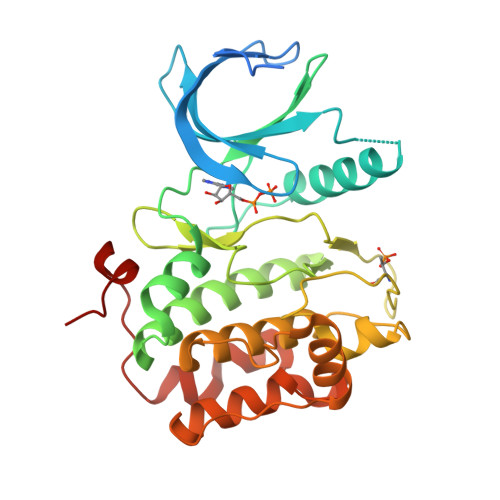
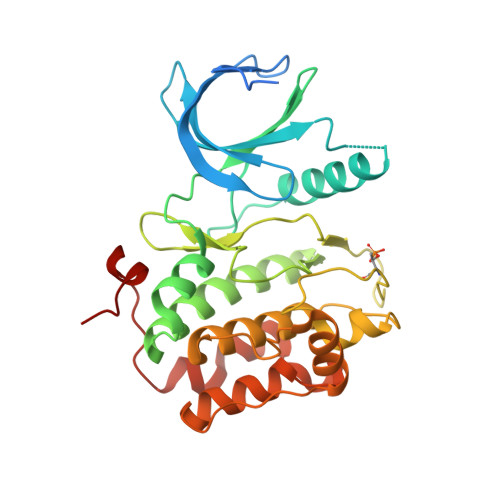
Structures of the wild-type and activated catalytic domains of Brachydanio rerio Polo-like kinase 1 (Plk1): changes in the active-site conformation and interactions with ligands.
Elling, R.A., Fucini, R.V., Romanowski, M.J.(2008) Acta Crystallogr D Biol Crystallogr 64: 909-918
- PubMed: 18703838
- DOI: https://doi.org/10.1107/S0907444908019513
- Primary Citation of Related Structures:
3D5U, 3D5V, 3D5W, 3D5X - PubMed Abstract:
Polo-like kinase 1 (Plk1) is a member of a family of serine/threonine kinases involved in the regulation of cell-cycle progression and cytokinesis and is an attractive target for the development of anticancer therapeutics. A zebrafish homolog of the human Plk1 (hPlk1) kinase domain (KD) was identified that can be expressed in large quantities in bacteria and crystallizes readily, whether in a wild-type form or as a variant containing the activating Thr196-->Asp substitution, in one space group and under similar conditions both in the absence and presence of active-site compounds. This construct was validated by testing a panel of hPlk1 inhibitors against human and zebrafish proteins and it was shown that the selected small molecules inhibited the homologs with a high degree of correlation. Crystal structures of ligand-free wild-type and activated zebrafish Plk1 (zPlk1) KDs revealed the organization of the secondary structural elements around the active site and demonstrated that the activation segment was disordered in the activated form of the domain but possessed a well defined secondary structure in the wild-type enzyme. The cocrystal structure of wild-type zPlk1 KD with ADP documented the hydrolysis of ATP and revealed the phosphorylation site. The cocrystal structure of the activated KD with wortmannin, a covalent inhibitor of Plk1 and PI3 kinases, showed the binding mode of the small molecule to the enzyme and may facilitate the design of more potent Plk1 inhibitors. The work presented in this study establishes the zPlk1 KD as a useful tool for rapid low- and high-throughput structure-based screening and drug discovery of compounds specific for this mitotic target.
Organizational Affiliation:
Department of Protein Sciences and Structural Biology, Sunesis Pharmaceuticals Inc., USA.