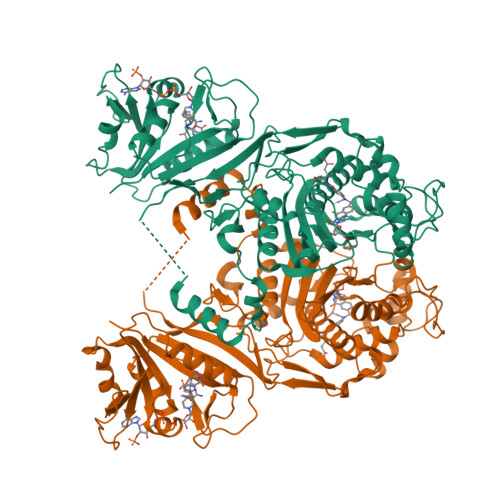
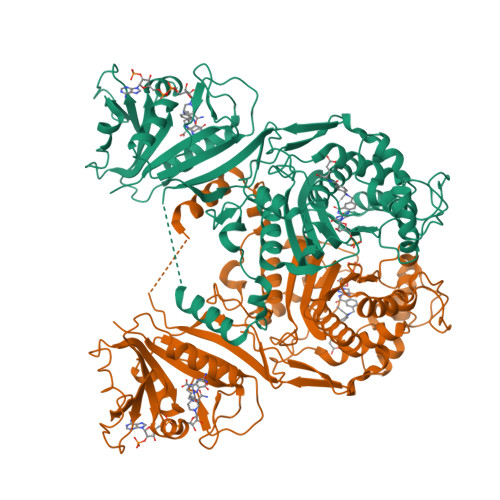
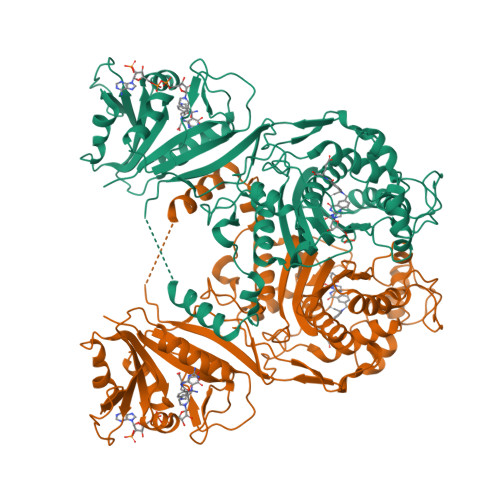
Explaining an unusually fast parasitic enzyme: folate tail-binding residues dictate substrate positioning and catalysis in Cryptosporidium hominis thymidylate synthase.
Martucci, W.E., Vargo, M.A., Anderson, K.S.(2008) Biochemistry 47: 8902-8911
- PubMed: 18672899
- DOI: https://doi.org/10.1021/bi800466z
- Primary Citation of Related Structures:
3DL5, 3DL6 - PubMed Abstract:
The essential enzyme TS-DHFR from Cryptosporidium hominis undergoes an unusually rapid rate of catalysis at the conserved TS domain, facilitated by two nonconserved residues, Ala287 and Ser290, in the folate tail-binding region. Mutation of these two residues to their conserved counterparts drastically affects multiple steps of the TS catalytic cycle. We have determined the crystal structures of all three mutants (A287F, S290G, and A287F/S290G) in complex with active site ligands dUMP and CB3717. The structural data show two effects of the mutations: an increased distance between the ligands in the active site and increased flexibility of the folate ligand in the partially open enzyme state that precedes conformational change to the active catalytic state. The latter effect is able to be rescued by the mutants containing the A287F mutation. In addition, the conserved water network of TS is altered in each of the mutants. The structural results point to a role of the folate tail-binding residues in closely positioning ChTS ligands and restricting ligand flexibility in the partially open state to allow for a rapid transition to the active closed state and enhanced rate of catalysis. These results provide an explanation on how folate tail-binding residues at one end of the active site affect long-range interactions throughout the TS active site and validate these residues as targets for species-specific drug design.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale University School of Medicine, 333 Cedar Street, New Haven, Connecticut 06520, USA.