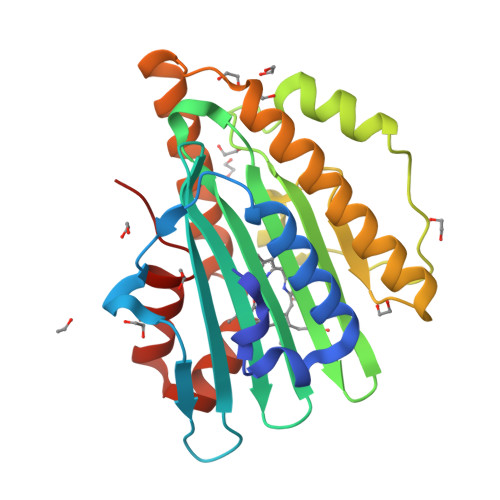
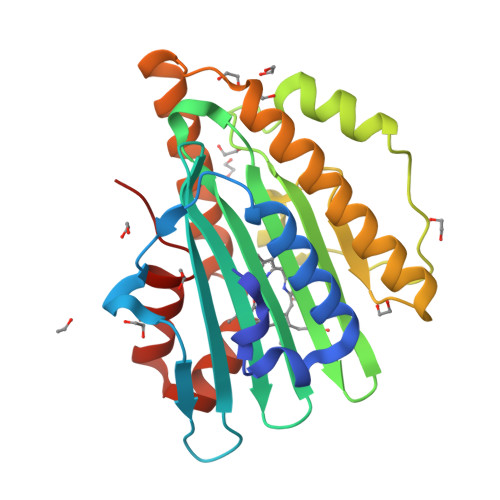
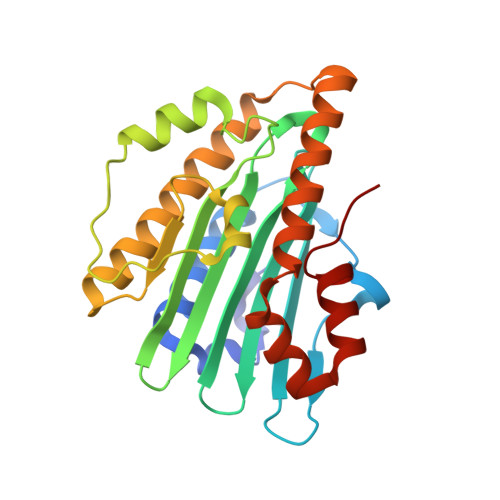
Structural basis for hydration dynamics in radical stabilization of bilin reductase mutants.
Kohler, A.C., Gae, D.D., Richley, M.A., Stoll, S., Gunn, A., Lim, S., Martin, S.S., Doukov, T.I., Britt, R.D., Ames, J.B., Lagarias, J.C., Fisher, A.J.(2010) Biochemistry 49: 6206-6218
- PubMed: 20557110
- DOI: https://doi.org/10.1021/bi100728q
- Primary Citation of Related Structures:
3F0L, 3F0M, 3NB8, 3NB9 - PubMed Abstract:
Heme-derived linear tetrapyrroles (phytobilins) in phycobiliproteins and phytochromes perform critical light-harvesting and light-sensing roles in oxygenic photosynthetic organisms. A key enzyme in their biogenesis, phycocyanobilin:ferredoxin oxidoreductase (PcyA), catalyzes the overall four-electron reduction of biliverdin IXalpha to phycocyanobilin--the common chromophore precursor for both classes of biliproteins. This interconversion occurs via semireduced bilin radical intermediates that are profoundly stabilized by selected mutations of two critical catalytic residues, Asp105 and His88. To understand the structural basis for this stabilization and to gain insight into the overall catalytic mechanism, we report the high-resolution crystal structures of substrate-loaded Asp105Asn and His88Gln mutants of Synechocystis sp. PCC 6803 PcyA in the initial oxidized and one-electron reduced radical states. Unlike wild-type PcyA, both mutants possess a bilin-interacting axial water molecule that is ejected from the active site upon formation of the enzyme-bound neutral radical complex. Structural studies of both mutants also show that the side chain of Glu76 is unfavorably located for D-ring vinyl reduction. On the basis of these structures and companion (15)N-(1)H long-range HMQC NMR analyses to assess the protonation state of histidine residues, we propose a new mechanistic scheme for PcyA-mediated reduction of both vinyl groups of biliverdin wherein an axial water molecule, which prematurely binds and ejects from both mutants upon one electron reduction, is required for catalytic turnover of the semireduced state.
Organizational Affiliation:
Department of Chemistry, University of California, One Shields Avenue, Davis, California 95616, USA.