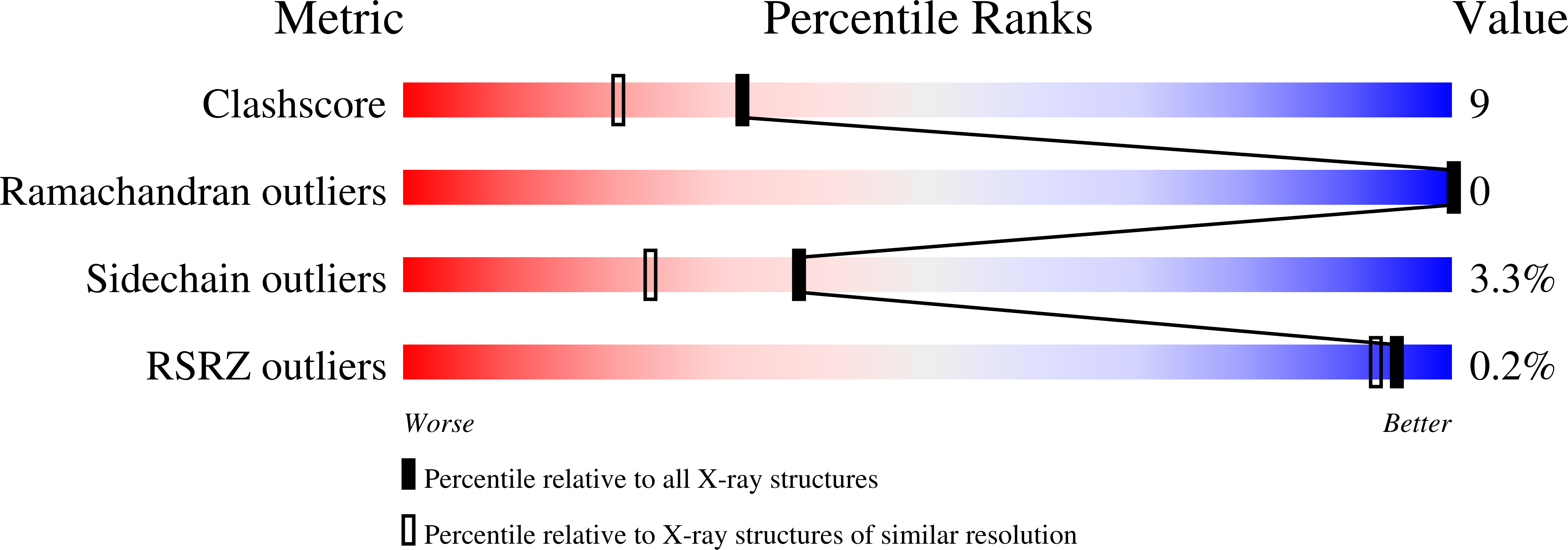
Structural and Functional Studies of QdtC: an N-Acetyltransferase Required for the Biosynthesis of dTDP-3-Acetamido-3,6-Dideoxy-alpha-D-Glucose.
Thoden, J., Cook, P., Schaffer, C., Messner, P., Holden, H.(2009) Biochemistry 48: 2699-2709
- PubMed: 19191736
- DOI: https://doi.org/10.1021/bi802313n
- Primary Citation of Related Structures:
3FS8, 3FSB, 3FSC - PubMed Abstract:
3-Acetamido-3,6-dideoxy-alpha-D-glucose or Quip3NAc is an unusual dideoxy sugar found in the O-antigens of various Gram-negative bacteria and in the S-layer glycoprotein glycans of some Gram-positive bacteria. It is produced in these organisms as a dTDP-linked sugar, with five enzymes ultimately required for its biosynthesis. The focus of this investigation is on the enzyme QdtC, a CoA-dependent N-acetyltransferase that catalyzes the last step in the Quip3NAc biosynthetic pathway. For this analysis, three crystal structures were determined: the wild-type enzyme in the presence of acetyl-CoA and two ternary complexes of the enzyme with CoA and either dTDP-D-Quip3N or dTDP-3-amino-3,6-didexoy-alpha-D-galactose (dTDP-D-Fucp3N). Each subunit of the trimeric enzyme is dominated by a left-handed beta-helix motif with 11 turns. The three active sites are located at the subunit-subunit interfaces, and the two dTDP-sugar ligands employed in this study bind to the protein in nearly identical manners. Those residues responsible for anchoring the hexose moieties of the dTDP-sugars to the protein include Glu 141, Asn 159, and Asp 160 from one subunit and His 134 from another subunit. To probe the roles of various amino acid residues in the catalytic mechanism of the enzyme, 10 site-directed mutant proteins were constructed and their kinetic parameters measured. On the basis of these data, a catalytic mechanism is proposed for QdtC in which the acetylation of the sugar amino group does not require a catalytic base provided by the protein. Rather, the sulfur of CoA functions as the ultimate proton acceptor.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin, Madison, Wisconsin 53706, USA.