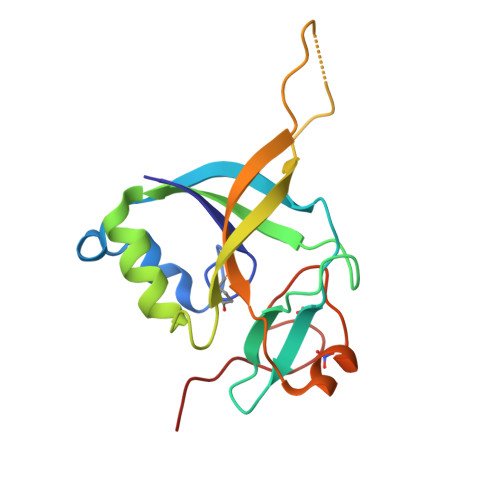
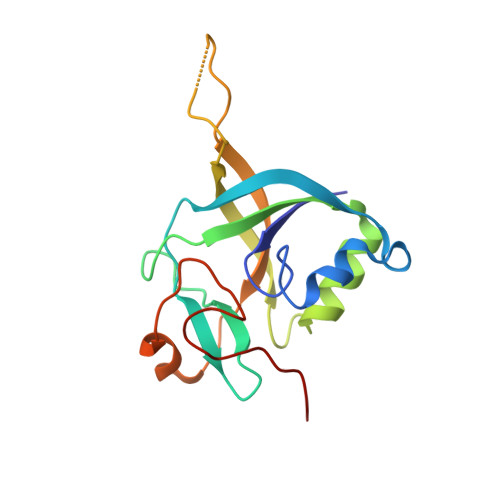
Identification and characterization of {gamma}-glutamylamine cyclotransferase: An enzyme responsible for {gamma}-glutamyl-{epsilon}-lysine catabolism
Oakley, A.J., Coggan, M., Board, P.G.(2010) J Biological Chem 285: 9642-9648
- PubMed: 20110353
- DOI: https://doi.org/10.1074/jbc.M109.082099
- Primary Citation of Related Structures:
3JUB, 3JUC, 3JUD - PubMed Abstract:
Gamma-glutamylamine cyclotransferase (GGACT) is an enzyme that converts gamma-glutamylamines to free amines and 5-oxoproline. GGACT shows high activity toward gamma-glutamyl-epsilon-lysine, derived from the breakdown of fibrin and other proteins cross-linked by transglutaminases. The enzyme adopts the newly identified cyclotransferase fold, observed in gamma-glutamylcyclotransferase (GGCT), an enzyme with activity toward gamma-glutamyl-alpha-amino acids (Oakley, A. J., Yamada, T., Liu, D., Coggan, M., Clark, A. G., and Board, P. G. (2008) J. Biol. Chem. 283, 22031-22042). Despite the absence of significant sequence identity, several residues are conserved in the active sites of GGCT and GGACT, including a putative catalytic acid/base residue (GGACT Glu(82)). The structure of GGACT in complex with the reaction product 5-oxoproline provides evidence for a common catalytic mechanism in both enzymes. The proposed mechanism, combined with the three-dimensional structures, also explains the different substrate specificities of these enzymes. Despite significant sequence divergence, there are at least three subfamilies in prokaryotes and eukaryotes that have conserved the GGCT fold and GGCT enzymatic activity.
Organizational Affiliation:
Division of Molecular and Health Technologies, Commonwealth Scientific and Industrial Research Organization, Parkville, Victoria 3052.