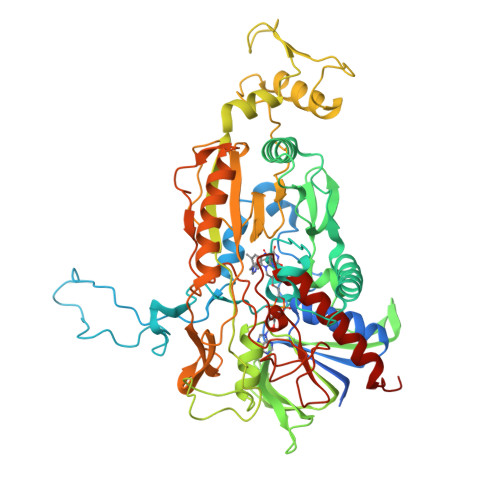
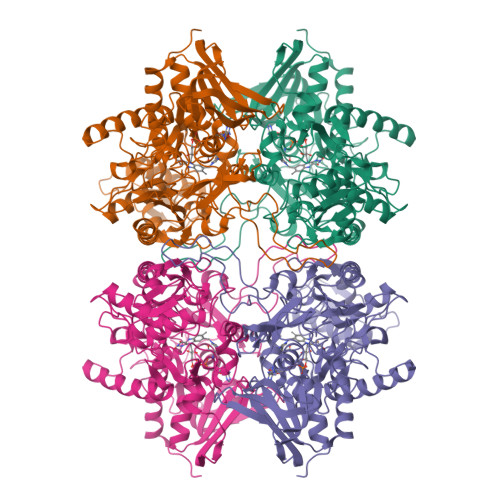
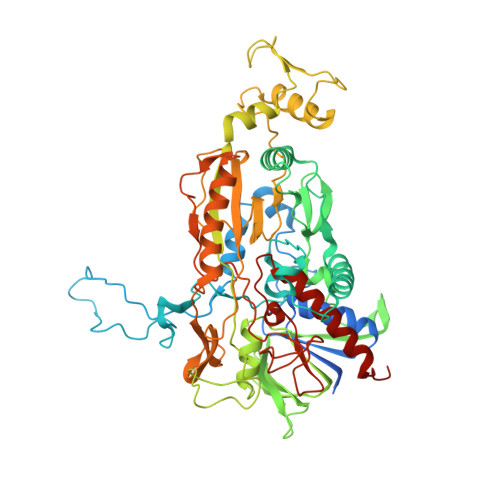
A conserved active-site threonine is important for both sugar and flavin oxidations of pyranose 2-oxidase.
Pitsawong, W., Sucharitakul, J., Prongjit, M., Tan, T.C., Spadiut, O., Haltrich, D., Divne, C., Chaiyen, P.(2010) J Biological Chem 285: 9697-9705
- PubMed: 20089849
- DOI: https://doi.org/10.1074/jbc.M109.073247
- Primary Citation of Related Structures:
3K4B, 3K4C - PubMed Abstract:
Pyranose 2-oxidase (P2O) catalyzes the oxidation by O(2) of d-glucose and several aldopyranoses to yield the 2-ketoaldoses and H(2)O(2). Based on crystal structures, in one rotamer conformation, the threonine hydroxyl of Thr(169) forms H-bonds to the flavin-N5/O4 locus, whereas, in a different rotamer, it may interact with either sugar or other parts of the P2O.sugar complex. Transient kinetics of wild-type (WT) and Thr(169) --> S/N/G/A replacement variants show that D-Glc binds to T169S, T169N, and WT with the same K(d) (45-47 mm), and the hydride transfer rate constants (k(red)) are similar (15.3-9.7 s(-1) at 4 degrees C). k(red) of T169G with D-glucose (0.7 s(-1), 4 degrees C) is significantly less than that of WT but not as severely affected as in T169A (k(red) of 0.03 s(-1) at 25 degrees C). Transient kinetics of WT and mutants using d-galactose show that P2O binds d-galactose with a one-step binding process, different from binding of d-glucose. In T169S, T169N, and T169G, the overall turnover with d-Gal is faster than that of WT due to an increase of k(red). In the crystal structure of T169S, Ser(169) O gamma assumes a position identical to that of O gamma 1 in Thr(169); in T169G, solvent molecules may be able to rescue H-bonding. Our data suggest that a competent reductive half-reaction requires a side chain at position 169 that is able to form an H-bond within the ES complex. During the oxidative half-reaction, all mutants failed to stabilize a C4a-hydroperoxyflavin intermediate, thus suggesting that the precise position and geometry of the Thr(169) side chain are required for intermediate stabilization.
Organizational Affiliation:
Department of Biochemistry and Center of Excellence in Protein Structure and Function, Faculty of Science, Mahidol University, Bangkok 10400, Thailand.