pH Dependence of Catalysis by Pseudomonas aeruginosa Isochorismate-Pyruvate Lyase: Implications for Transition State Stabilization and the Role of Lysine 42.
Olucha, J., Ouellette, A.N., Luo, Q., Lamb, A.L.(2011) Biochemistry 50: 7198-7207
- PubMed: 21751784
- DOI: https://doi.org/10.1021/bi200599j
- Primary Citation of Related Structures:
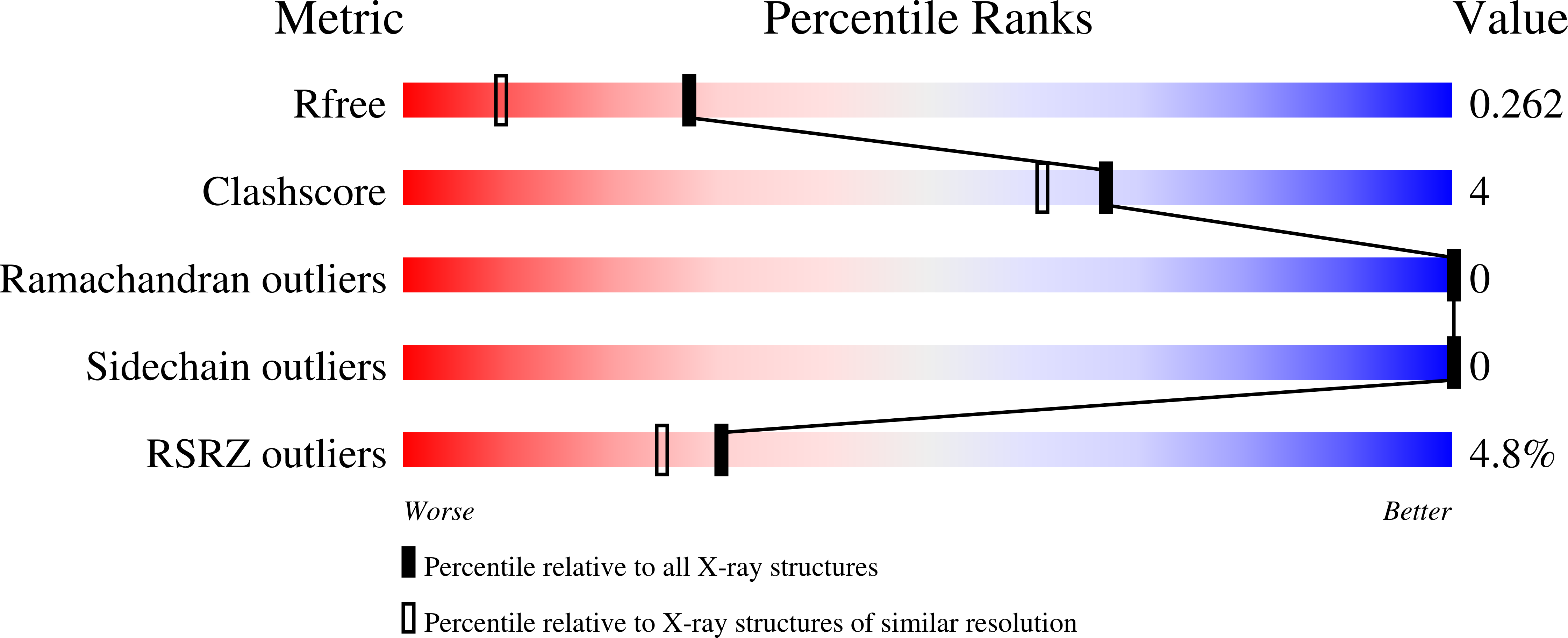
3REM, 3RET - PubMed Abstract:
An isochorismate-pyruvate lyase with adventitious chorismate mutase activity from Pseudomonas aerugionsa (PchB) achieves catalysis of both pericyclic reactions in part by the stabilization of reactive conformations and in part by electrostatic transition-state stabilization. When the active site loop Lys42 is mutated to histidine, the enzyme develops a pH dependence corresponding to a loss of catalytic power upon deprotonation of the histidine. Structural data indicate that the change is not due to changes in active site architecture, but due to the difference in charge at this key site. With loss of the positive charge on the K42H side chain at high pH, the enzyme retains lyase activity at ∼100-fold lowered catalytic efficiency but loses detectable mutase activity. We propose that both substrate organization and electrostatic transition state stabilization contribute to catalysis. However, the dominant reaction path for catalysis is dependent on reaction conditions, which influence the electrostatic properties of the enzyme active site amino acid side chains.
Organizational Affiliation:
Department of Molecular Biosciences, University of Kansas, Lawrence, Kansas 66045, USA.