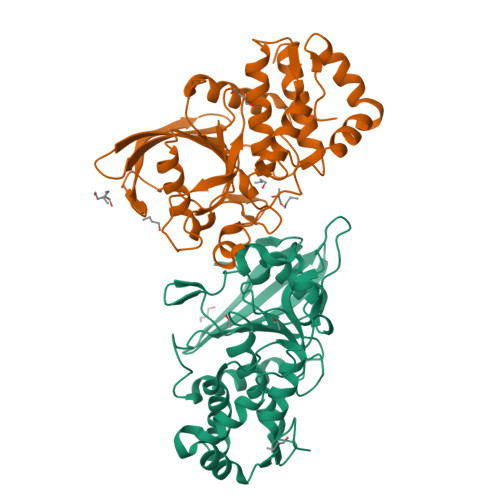
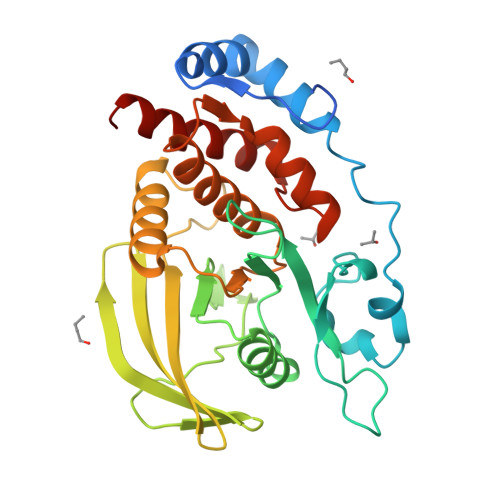
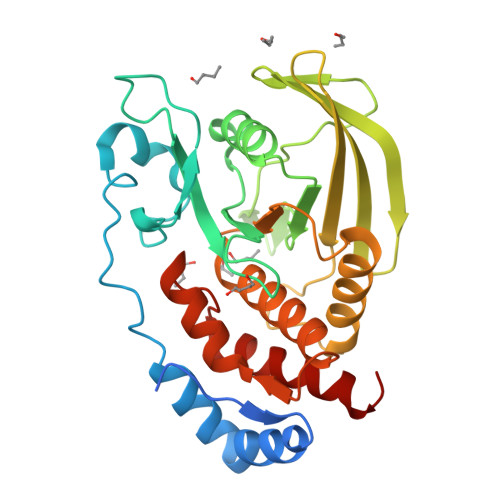
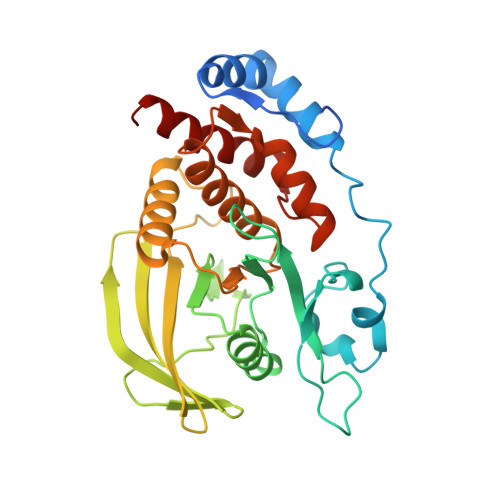
Conformational basis for substrate recruitment in protein tyrosine phosphatase 10D
Madan, L.L., Gopal, B.(2011) Biochemistry 50: 10114-10125
- PubMed: 22007620
- DOI: https://doi.org/10.1021/bi201092q
- Primary Citation of Related Structures:
3S3E, 3S3F, 3S3H, 3S3K - PubMed Abstract:
The coordinated activity of protein tyrosine phosphatases (PTPs) is crucial for the initiation, modulation, and termination of diverse cellular processes. The catalytic activity of this protein depends on a nucleophilic cysteine at the active site that mediates the hydrolysis of the incoming phosphotyrosine substrate. While the role of conserved residues in the catalytic mechanism of PTPs has been extensively examined, the diversity in the mechanisms of substrate recognition and modulation of catalytic activity suggests that other, less conserved sequence and structural features could contribute to this process. Here we describe the crystal structures of Drosophila melanogaster PTP10D in the apo form as well as in a complex with a substrate peptide and an inhibitor. These studies reveal the role of aromatic ring stacking interactions at the boundary of the active site of PTPs in mediating substrate recruitment. We note that phenylalanine 76, of the so-called KNRY loop, is crucial for orienting the phosphotyrosine residue toward the nucleophilic cysteine. Mutation of phenylalanine 76 to leucine results in a 60-fold decrease in the catalytic efficiency of the enzyme. Fluorescence measurements with a competitive inhibitor, p-nitrocatechol sulfate, suggest that Phe76 also influences the formation of the enzyme-substrate intermediate. The structural and biochemical data for PTP10D thus highlight the role of relatively less conserved residues in PTP domains in both substrate recruitment and modulation of reaction kinetics.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, India.