Cloning, expression, purification, crystallization and X-ray diffraction analysis of dihydrodipicolinate synthase from the human pathogenic bacterium Bartonella henselae strain Houston-1 at 2.1 angstrom resolution.
Naqvi, K.F., Staker, B.L., Dobson, R.C., Serbzhinskiy, D., Sankaran, B., Myler, P.J., Hudson, A.O.(2016) Acta Crystallogr F Struct Biol Commun 72: 2-9
- PubMed: 26750477
- DOI: https://doi.org/10.1107/S2053230X15023213
- Primary Citation of Related Structures:
3SI9 - PubMed Abstract:
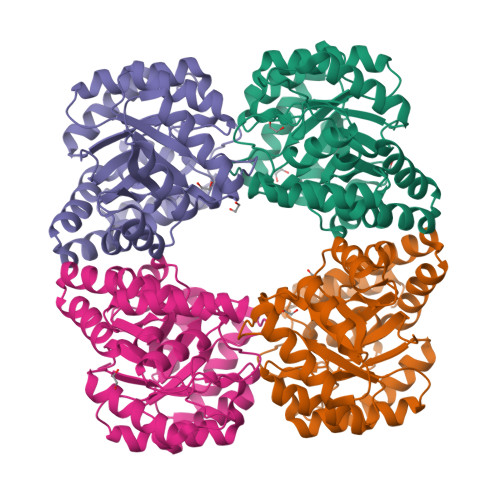
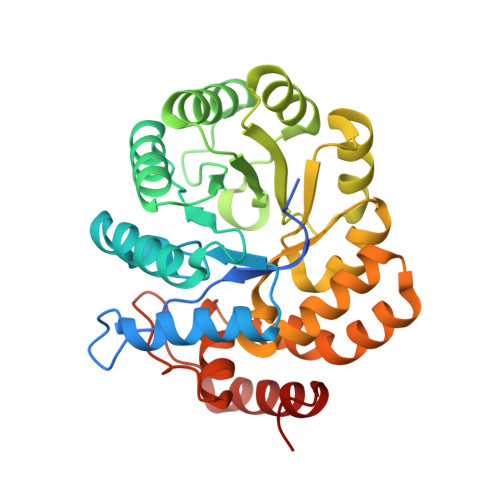
The enzyme dihydrodipicolinate synthase catalyzes the committed step in the synthesis of diaminopimelate and lysine to facilitate peptidoglycan and protein synthesis. Dihydrodipicolinate synthase catalyzes the condensation of L-aspartate 4-semialdehyde and pyruvate to synthesize L-2,3-dihydrodipicolinate. Here, the cloning, expression, purification, crystallization and X-ray diffraction analysis of dihydrodipicolinate synthase from the pathogenic bacterium Bartonella henselae, the causative bacterium of cat-scratch disease, are presented. Protein crystals were grown in conditions consisting of 20%(w/v) PEG 4000, 100 mM sodium citrate tribasic pH 5.5 and were shown to diffract to ∼2.10 Å resolution. They belonged to space group P212121, with unit-cell parameters a = 79.96, b = 106.33, c = 136.25 Å. The final R values were Rr.i.m. = 0.098, Rwork = 0.183, Rfree = 0.233.
Organizational Affiliation:
Thomas H. Gosnell School of Life Sciences, Rochester Institute of Technology, 85 Lomb Memorial Drive, Rochester, NY 14623-5603, USA.