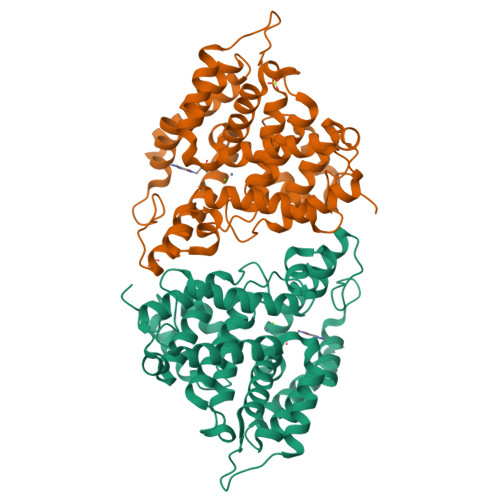
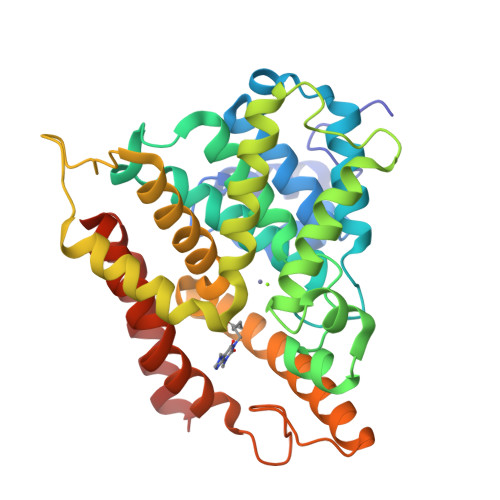
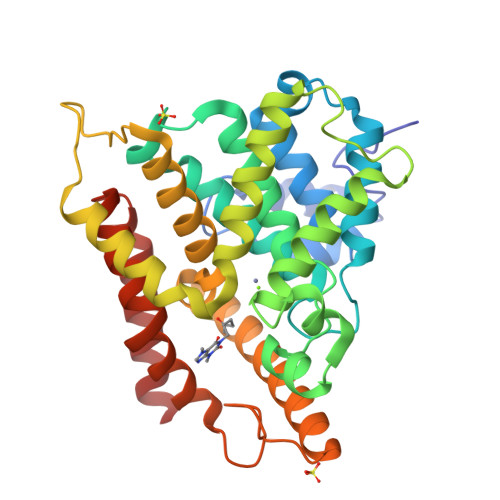
Fragment-Based Screening for Inhibitors of PDE4A Using Enthalpy Arrays and X-ray Crystallography.
Recht, M.I., Sridhar, V., Badger, J., Hernandez, L., Chie-Leon, B., Nienaber, V., Torres, F.E.(2012) J Biomol Screen 17: 469-480
- PubMed: 22223051
- DOI: https://doi.org/10.1177/1087057111430987
- Primary Citation of Related Structures:
3TVX - PubMed Abstract:
Fragment-based screening has typically relied on X-ray or nuclear magnetic resonance methods to identify low-affinity ligands that bind to therapeutic targets. These techniques are expensive in terms of material and time, so it useful to have a higher throughput method to reliably prescreen a fragment library to identify a subset of compounds for structural analysis. Calorimetry provides a label-free method to assay binding and enzymatic activity that is unaffected by the spectroscopic properties of the sample. Conventional microcalorimetry is hampered by requiring large quantities of reagents and long measurement times. Nanocalorimeters can overcome these limitations of conventional isothermal titration calorimetry. Here we have used enthalpy arrays, which are arrays of nanocalorimeters, to perform an enzyme activity-based fragment screen for competitive inhibitors of phosphodiesterase 4A (PDE4A). Several inhibitors with K ( I ) <2 mM were identified and moved to X-ray crystallization trials. Although the co-crystals did not yield high-resolution data, evidence of binding was observed, and the chemical structures of the hits were consistent with motifs of known PDE4 inhibitors. This study shows how array calorimetry can be used as a prescreening method for fragment-based lead discovery with enzyme targets and provides a list of candidate fragments for inhibition of PDE4A.
Organizational Affiliation:
Palo Alto Research Center, Palo Alto, CA, USA. michael.recht@parc.com