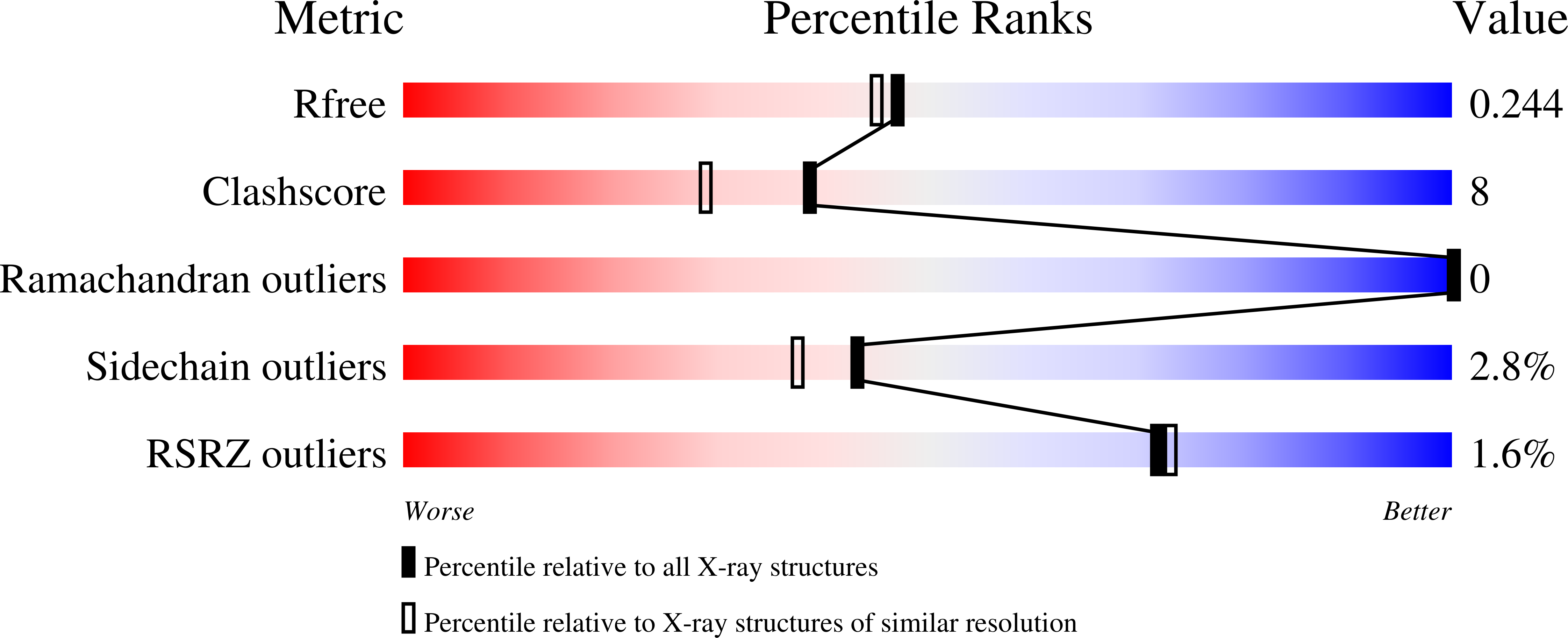
The loss of an electrostatic contact unique to AMPA receptor ligand binding domain 2 slows channel activation.
Holley, S.M., Ahmed, A.H., Srinivasan, J., Murthy, S.E., Weiland, G.A., Oswald, R.E., Nowak, L.M.(2012) Biochemistry 51: 4015-4027
- PubMed: 22512472
- DOI: https://doi.org/10.1021/bi3001837
- Primary Citation of Related Structures:
4F1Y, 4F22, 4F29, 4F2O, 4F2Q, 4F31, 4F39, 4F3B, 4F3G - PubMed Abstract:
Ligand-gated ion channels undergo conformational changes that transfer the energy of agonist binding to channel opening. Within ionotropic glutamate receptor (iGluR) subunits, this process is initiated in their bilobate ligand binding domain (LBD) where agonist binding to lobe 1 favors closure of lobe 2 around the agonist and allows formation of interlobe hydrogen bonds. AMPA receptors (GluAs) differ from other iGluRs because glutamate binding causes an aspartate-serine peptide bond in a flexible part of lobe 2 to rotate 180° (flipped conformation), allowing these residues to form cross-cleft H-bonds with tyrosine and glycine in lobe 1. This aspartate also contacts the side chain of a lysine residue in the hydrophobic core of lobe 2 by a salt bridge. We investigated how the peptide flip and electrostatic contact (D655-K660) in GluA3 contribute to receptor function by examining pharmacological and structural properties with an antagonist (CNQX), a partial agonist (kainate), and two full agonists (glutamate and quisqualate) in the wildtype and two mutant receptors. Alanine substitution decreased the agonist potency of GluA3(i)-D655A and GluA3(i)-K660A receptor channels expressed in HEK293 cells and differentially affected agonist binding affinity for isolated LBDs without changing CNQX affinity. Correlations observed in the crystal structures of the mutant LBDs included the loss of the D655-K660 electrostatic contact, agonist-dependent differences in lobe 1 and lobe 2 closure, and unflipped D(A)655-S656 bonds. Glutamate-stimulated activation was slower for both mutants, suggesting that efficient energy transfer of agonist binding within the LBD of AMPA receptors requires an intact tether between the flexible peptide flip domain and the rigid hydrophobic core of lobe 2.
Organizational Affiliation:
Department of Molecular Medicine, Cornell University, Ithaca, NY 14853, USA.