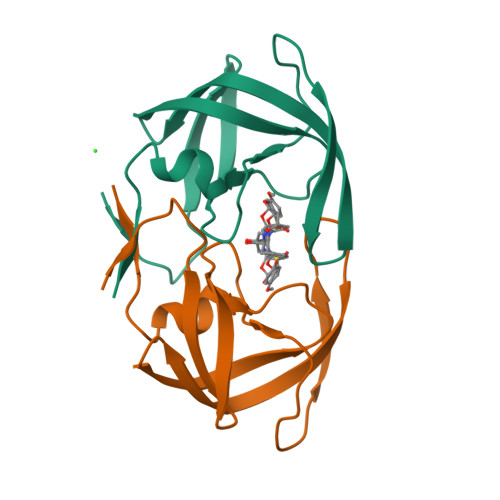
Novel P2 tris-tetrahydrofuran group in antiviral compound 1 (GRL-0519) fills the S2 binding pocket of selected mutants of HIV-1 protease.
Zhang, H., Wang, Y.F., Shen, C.H., Agniswamy, J., Rao, K.V., Xu, C.X., Ghosh, A.K., Harrison, R.W., Weber, I.T.(2013) J Med Chem 56: 1074-1083
- PubMed: 23298236
- DOI: https://doi.org/10.1021/jm301519z
- Primary Citation of Related Structures:
4HDB, 4HDF, 4HDP, 4HE9, 4HEG - PubMed Abstract:
GRL-0519 (1) is a potent antiviral inhibitor of HIV-1 protease (PR) possessing tris-tetrahydrofuran (tris-THF) at P2. The high resolution X-ray crystal structures of inhibitor 1 in complexes with single substitution mutants PR(R8Q), PR(D30N), PR(I50V), PR(I54M), and PR(V82A) were analyzed in relation to kinetic data. The smaller valine side chain in PR(I50V) eliminated hydrophobic interactions with inhibitor and the other subunit consistent with 60-fold worse inhibition. Asn30 in PR(D30N) showed altered interactions with neighboring residues and 18-fold worse inhibition. Mutations V82A and I54M showed compensating structural changes consistent with 6-7-fold lower inhibition. Gln8 in PR(R8Q) replaced the ionic interactions of wild type Arg8 with hydrogen bond interactions without changing the inhibition significantly. The carbonyl oxygen of Gly48 showed two alternative conformations in all structures likely due to the snug fit of the large tris-THF group in the S2 subsite in agreement with high antiviral efficacy of 1 on resistant virus.
Organizational Affiliation:
Department of Biology, Georgia State University, Atlanta, Georgia 30303, USA.