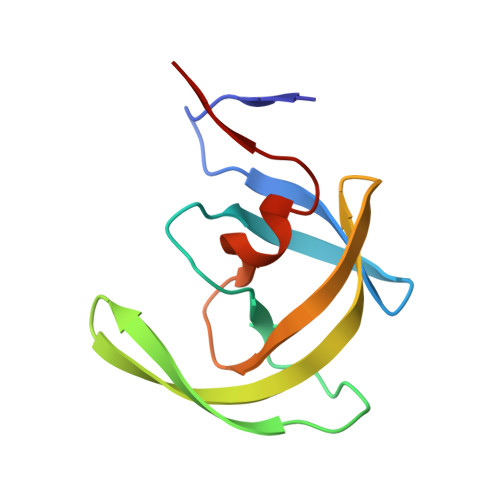
Structural basis and distal effects of Gag substrate coevolution in drug resistance to HIV-1 protease.
Ozen, A., Lin, K.H., Kurt Yilmaz, N., Schiffer, C.A.(2014) Proc Natl Acad Sci U S A 111: 15993-15998
- PubMed: 25355911
- DOI: https://doi.org/10.1073/pnas.1414063111
- Primary Citation of Related Structures:
4QJ2, 4QJ6, 4QJ7, 4QJ8, 4QJ9, 4QJA - PubMed Abstract:
Drug resistance mutations in response to HIV-1 protease inhibitors are selected not only in the drug target but elsewhere in the viral genome, especially at the protease cleavage sites in the precursor protein Gag. To understand the molecular basis of this protease-substrate coevolution, we solved the crystal structures of drug resistant I50V/A71V HIV-1 protease with p1-p6 substrates bearing coevolved mutations. Analyses of the protease-substrate interactions reveal that compensatory coevolved mutations in the substrate do not restore interactions lost due to protease mutations, but instead establish other interactions that are not restricted to the site of mutation. Mutation of a substrate residue has distal effects on other residues' interactions as well, including through the induction of a conformational change in the protease. Additionally, molecular dynamics simulations suggest that restoration of active site dynamics is an additional constraint in the selection of coevolved mutations. Hence, protease-substrate coevolution permits mutational, structural, and dynamic changes via molecular mechanisms that involve distal effects contributing to drug resistance.
Organizational Affiliation:
Department of Biochemistry and Molecular Pharmacology, University of Massachusetts Medical School, Worcester, MA 01605.