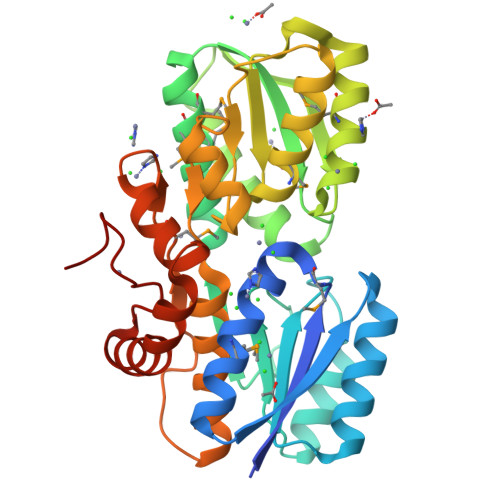
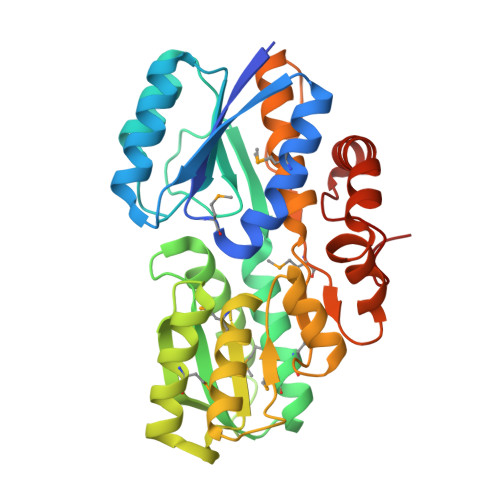
A General Strategy for the Discovery of Metabolic Pathways: d-Threitol, l-Threitol, and Erythritol Utilization in Mycobacterium smegmatis.
Huang, H., Carter, M.S., Vetting, M.W., Al-Obaidi, N., Patskovsky, Y., Almo, S.C., Gerlt, J.A.(2015) J Am Chem Soc 137: 14570-14573
- PubMed: 26560079
- DOI: https://doi.org/10.1021/jacs.5b08968
- Primary Citation of Related Structures:
4RS3, 4RSM - PubMed Abstract:
We describe a general integrated bioinformatic and experimental strategy to discover the in vitro enzymatic activities and in vivo functions (metabolic pathways) of uncharacterized enzymes discovered in microbial genome projects using the ligand specificities of the solute binding proteins (SBPs) for ABC transporters. Using differential scanning fluorimetry, we determined that the SBP for an ABC transporter encoded by the genome of Mycobacterium smegmatis is stabilized by d-threitol. Using sequence similarity networks and genome neighborhood networks to guide selection of target proteins for pathway enzymes, we applied both in vitro and in vivo experimental approaches to discover novel pathways for catabolism of d-threitol, l-threitol, and erythritol.
Organizational Affiliation:
Department of Biochemistry, Albert Einstein College of Medicine , Bronx, New York 10461, United States.