Crystal structure of mouse Xyloside xylosyltransferase 1 complexed with manganese,acceptor ligand and UDP-Xylose
Yu, H., Li, H.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Xyloside xylosyltransferase 1 | 306 | Mus musculus | Mutation(s): 0 Gene Names: Xxylt1 EC: 2.4.2 (PDB Primary Data), 2.4.2.62 (UniProt) | 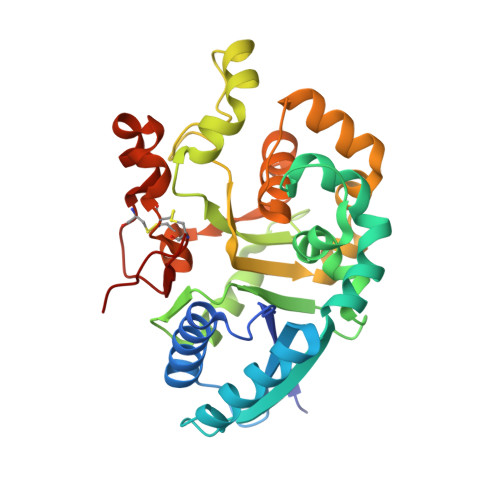 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q3U4G3 (Mus musculus) Explore Q3U4G3 Go to UniProtKB: Q3U4G3 | |||||
IMPC: MGI:2146443 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q3U4G3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Coagulation factor IX | B [auth D] | 50 | Homo sapiens | Mutation(s): 0 Gene Names: F9 EC: 3.4.21.22 | 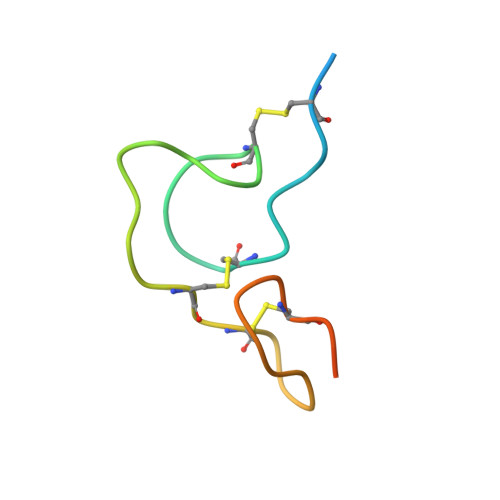 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00740 (Homo sapiens) Explore P00740 Go to UniProtKB: P00740 | |||||
PHAROS: P00740 GTEx: ENSG00000101981 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00740 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: P00740-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| UDX Query on UDX | E [auth A] | URIDINE-5'-DIPHOSPHATE-XYLOPYRANOSE C14 H22 N2 O16 P2 DQQDLYVHOTZLOR-OCIMBMBZSA-N |  | ||
| MN Query on MN | D [auth A] | MANGANESE (II) ION Mn WAEMQWOKJMHJLA-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 89.481 | α = 90 |
| b = 89.481 | β = 90 |
| c = 42.895 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |