Crystal structure and stability of gyrase-fluoroquinolone cleaved complexes from Mycobacterium tuberculosis.
Blower, T.R., Williamson, B.H., Kerns, R.J., Berger, J.M.(2016) Proc Natl Acad Sci U S A 113: 1706-1713
- PubMed: 26792525
- DOI: https://doi.org/10.1073/pnas.1525047113
- Primary Citation of Related Structures:
5BS8, 5BTA, 5BTC, 5BTD, 5BTF, 5BTG, 5BTI, 5BTL, 5BTN - PubMed Abstract:
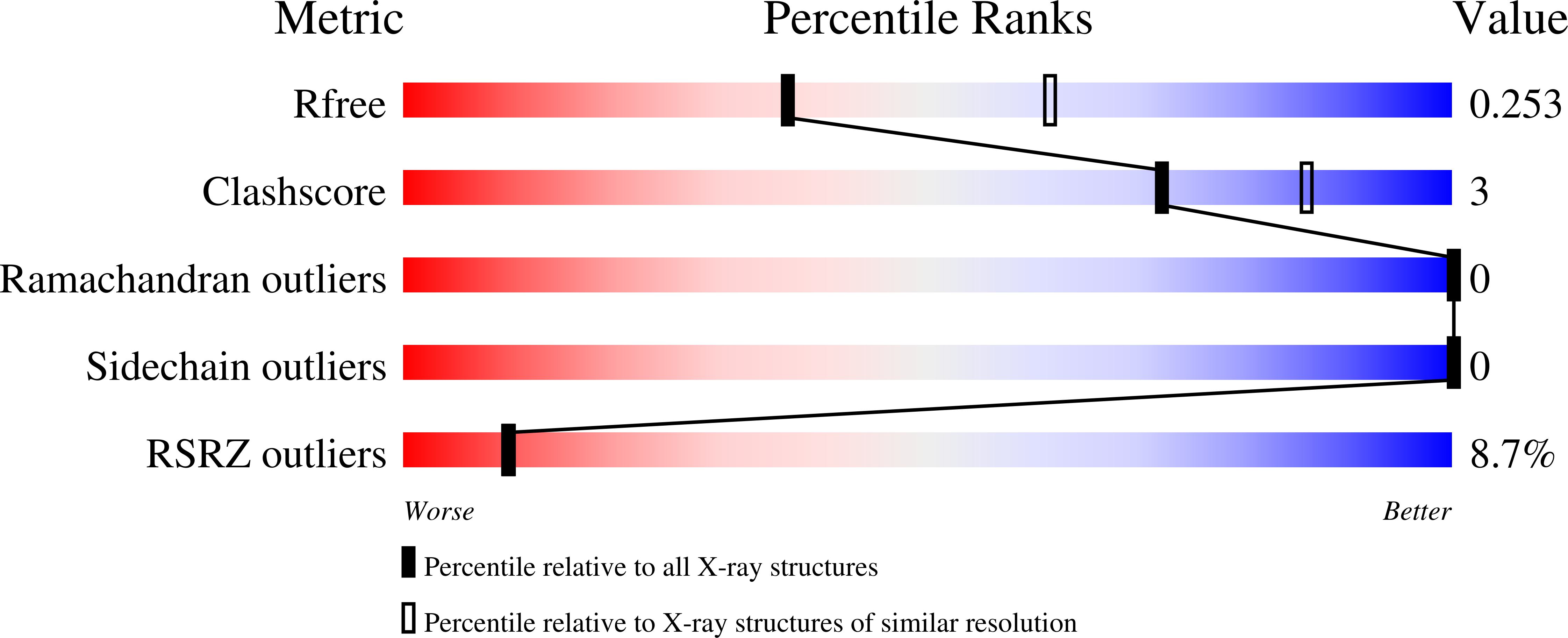
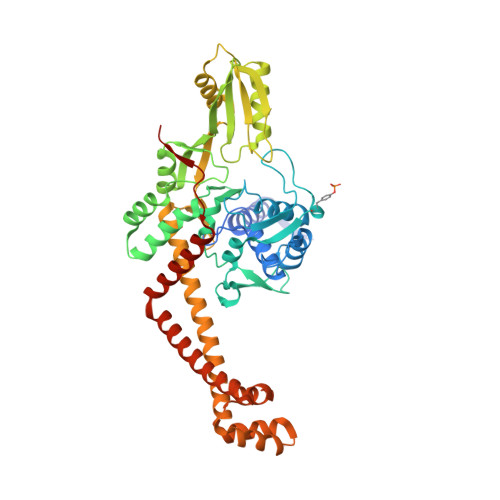
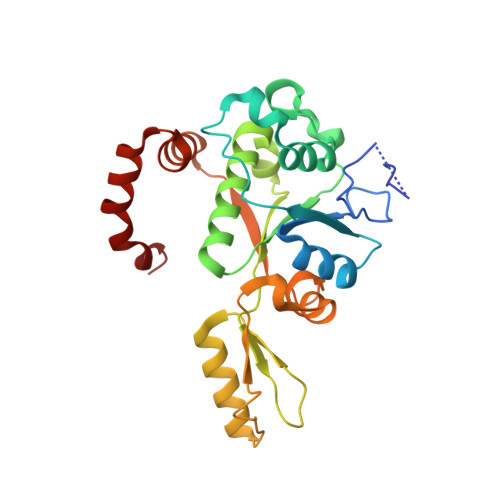
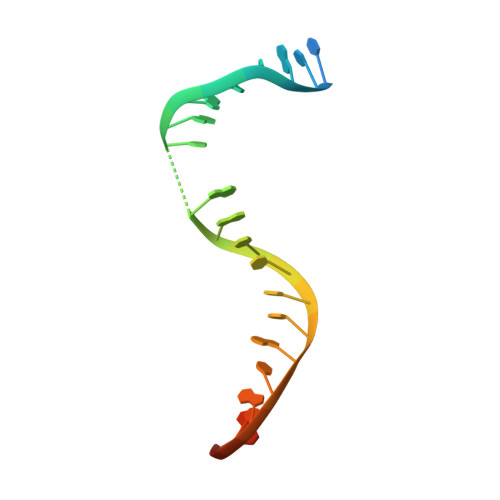
Mycobacterium tuberculosis (Mtb) infects one-third of the world's population and in 2013 accounted for 1.5 million deaths. Fluoroquinolone antibacterials, which target DNA gyrase, are critical agents used to halt the progression from multidrug-resistant tuberculosis to extensively resistant disease; however, fluoroquinolone resistance is emerging and new ways to bypass resistance are required. To better explain known differences in fluoroquinolone action, the crystal structures of the WT Mtb DNA gyrase cleavage core and a fluoroquinolone-sensitized mutant were determined in complex with DNA and five fluoroquinolones. The structures, ranging from 2.4- to 2.6-Å resolution, show that the intrinsically low susceptibility of Mtb to fluoroquinolones correlates with a reduction in contacts to the water shell of an associated magnesium ion, which bridges fluoroquinolone-gyrase interactions. Surprisingly, the structural data revealed few differences in fluoroquinolone-enzyme contacts from drugs that have very different activities against Mtb. By contrast, a stability assay using purified components showed a clear relationship between ternary complex reversibility and inhibitory activities reported with cultured cells. Collectively, our data indicate that the stability of fluoroquinolone/DNA interactions is a major determinant of fluoroquinolone activity and that moieties that have been appended to the C7 position of different quinolone scaffolds do not take advantage of specific contacts that might be made with the enzyme. These concepts point to new approaches for developing quinolone-class compounds that have increased potency against Mtb and the ability to overcome resistance.
Organizational Affiliation:
Department of Biophysics and Biophysical Chemistry, Johns Hopkins University School of Medicine, Baltimore, MD 21205;