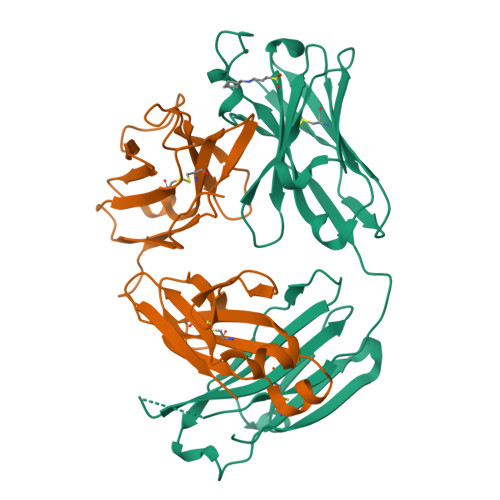
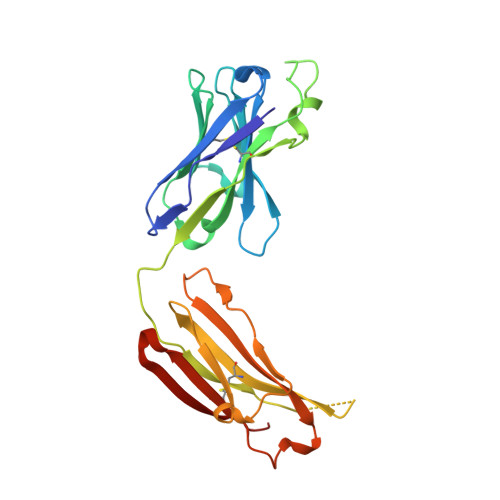
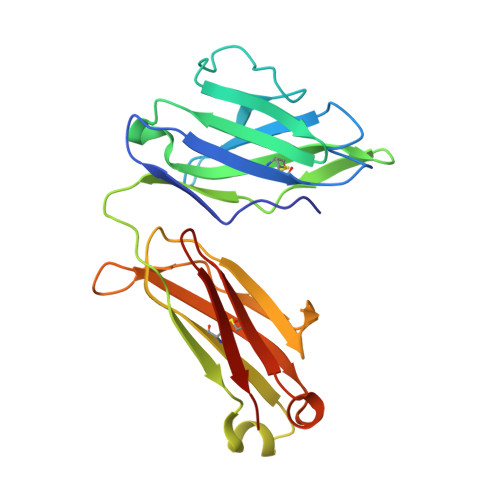
Antibodies to a conformational epitope on gp41 neutralize HIV-1 by destabilizing the Env spike.
Lee, J.H., Leaman, D.P., Kim, A.S., Torrents de la Pena, A., Sliepen, K., Yasmeen, A., Derking, R., Ramos, A., de Taeye, S.W., Ozorowski, G., Klein, F., Burton, D.R., Nussenzweig, M.C., Poignard, P., Moore, J.P., Klasse, P.J., Sanders, R.W., Zwick, M.B., Wilson, I.A., Ward, A.B.(2015) Nat Commun 6: 8167-8167
- PubMed: 26404402
- DOI: https://doi.org/10.1038/ncomms9167
- Primary Citation of Related Structures:
5AWN, 5CCK - PubMed Abstract:
The recent identification of three broadly neutralizing antibodies (bnAbs) against gp120-gp41 interface epitopes has expanded the targetable surface on the HIV-1 envelope glycoprotein (Env) trimer. By using biochemical, biophysical and computational methods, we map the previously unknown trimer epitopes of two related antibodies, 3BC315 and 3BC176. A cryo-EM reconstruction of a soluble Env trimer bound to 3BC315 Fab at 9.3 Å resolution reveals that the antibody binds between two gp41 protomers, and neutralizes the virus by accelerating trimer decay. In contrast, bnAb 35O22 binding to a partially overlapping quaternary epitope at the gp120-gp41 interface does not induce decay. A conserved gp41-proximal glycan at N88 was also shown to play a role in the binding kinetics of 3BC176 and 3BC315. Finally, our data suggest that the dynamic structure of the Env trimer influences exposure of bnAb epitopes.
Organizational Affiliation:
Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, California 92037, USA.