CAL PDZ domain with peptide and inhibitor
Zhao, Y., Madden, D.R.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Golgi-associated PDZ and coiled-coil motif-containing protein | 87 | Homo sapiens | Mutation(s): 0 Gene Names: GOPC, CAL, FIG | 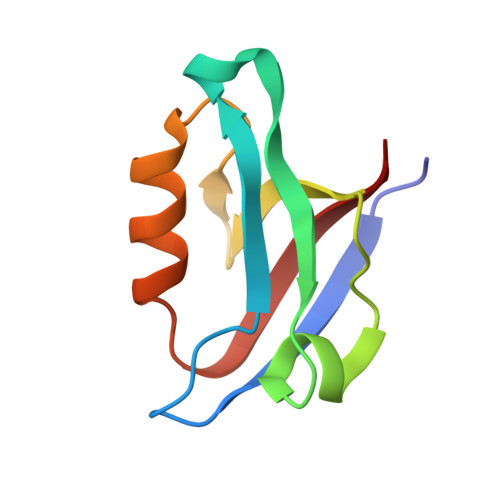 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9HD26 (Homo sapiens) Explore Q9HD26 Go to UniProtKB: Q9HD26 | |||||
PHAROS: Q9HD26 GTEx: ENSG00000047932 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9HD26 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| HPV18E6 Peptide | 10 | Alphapapillomavirus 7 | Mutation(s): 0 | 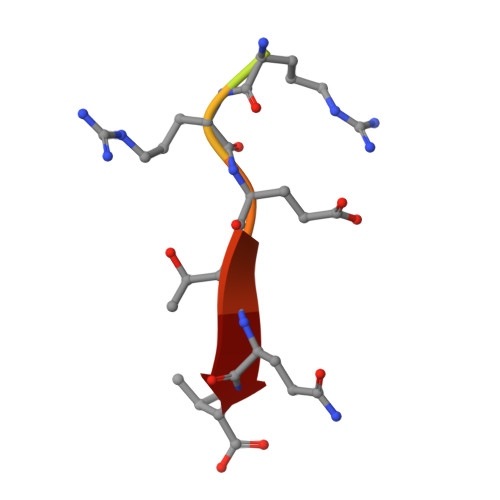 | |
UniProt | |||||
Find proteins for P06463 (Human papillomavirus type 18) Explore P06463 Go to UniProtKB: P06463 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P06463 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PQR Query on PQR | E [auth A] | 4-[methyl(nitroso)amino]benzene-1,2-diol C7 H8 N2 O3 XAKAQCMEMMZUEO-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 32.772 | α = 90 |
| b = 48.252 | β = 101.47 |
| c = 51.639 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data scaling |
| PHENIX | phasing |
| XDS | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease (NIH/NIDDK) | United States | R01-DK101541 |