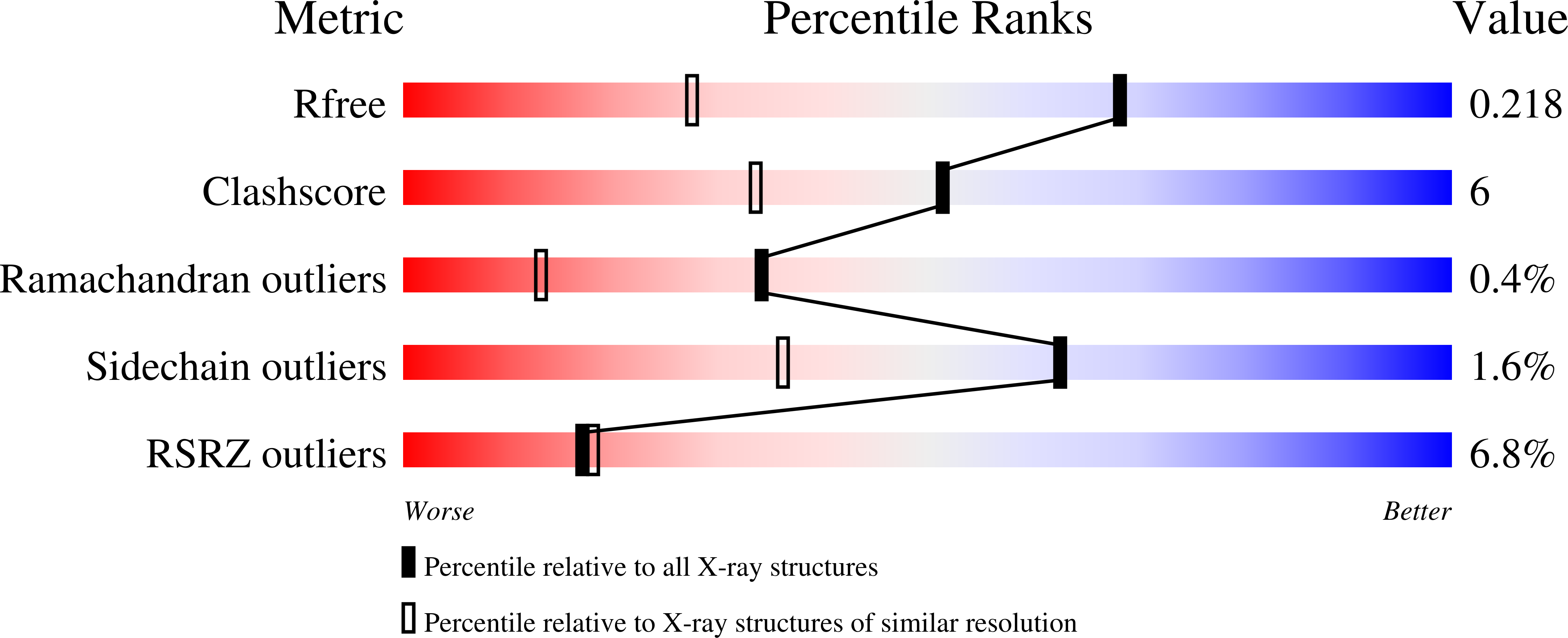
Conformational Change of a Tryptophan Residue in BtuF Facilitates Binding and Transport of Cobinamide by the Vitamin B12 Transporter BtuCD-F.
Mireku, S.A., Ruetz, M., Zhou, T., Korkhov, V.M., Krautler, B., Locher, K.P.(2017) Sci Rep 7: 41575-41575
- PubMed: 28128319
- DOI: https://doi.org/10.1038/srep41575
- Primary Citation of Related Structures:
5M29, 5M2Q, 5M34, 5M3B - PubMed Abstract:
BtuCD-F is an ABC transporter that mediates cobalamin uptake into Escherichia coli. Early in vivo data suggested that BtuCD-F might also be involved in the uptake of cobinamide, a cobalamin precursor. However, neither was it demonstrated that BtuCD-F indeed transports cobinamide, nor was the structural basis of its recognition known. We synthesized radiolabeled cyano-cobinamide and demonstrated BtuCD-catalyzed in vitro transport, which was ATP- and BtuF-dependent. The crystal structure of cobinamide-bound BtuF revealed a conformational change of a tryptophan residue (W66) in the substrate binding cleft compared to the structure of cobalamin-bound BtuF. High-affinity binding of cobinamide was dependent on W66, because mutation to most other amino acids substantially reduced binding. The structures of three BtuF W66 mutants revealed that tight packing against bound cobinamide was only provided by tryptophan and phenylalanine, in line with the observed binding affinities. In vitro transport rates of cobinamide and cobalamin were not influenced by the substitutions of BtuF W66 under the experimental conditions, indicating that W66 has no critical role in the transport reaction. Our data present the molecular basis of the cobinamide versus cobalamin specificity of BtuCD-F and provide tools for in vitro cobinamide transport and binding assays.
Organizational Affiliation:
Institute of Molecular Biology and Biophysics, Eidgenössische Technische Hochschule (ETH) Zürich, CH-8093 Zürich, Switzerland.