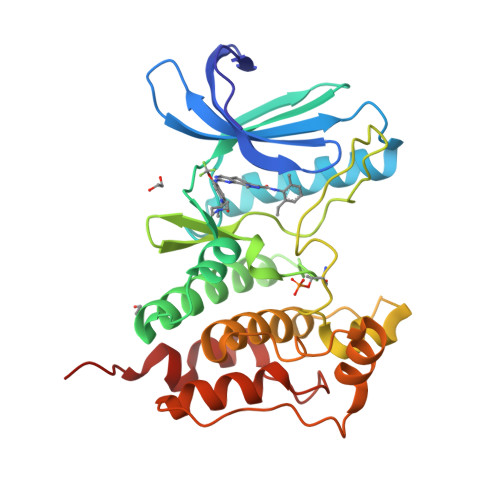
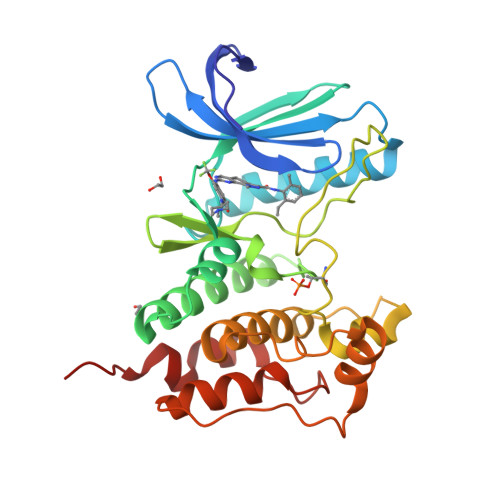
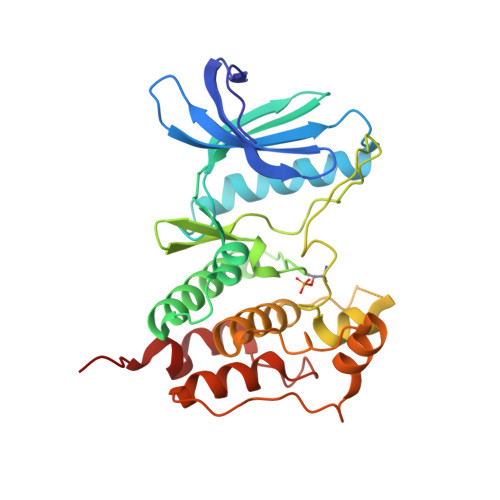
Understanding inhibitor resistance in Mps1 kinase through novel biophysical assays and structures.
Hiruma, Y., Koch, A., Hazraty, N., Tsakou, F., Medema, R.H., Joosten, R.P., Perrakis, A.(2017) J Biological Chem 292: 14496-14504
- PubMed: 28726638
- DOI: https://doi.org/10.1074/jbc.M117.783555
- Primary Citation of Related Structures:
5MRB, 5NTT, 5O91 - PubMed Abstract:
Monopolar spindle 1 (Mps1/TTK) is a protein kinase essential in mitotic checkpoint signaling, preventing anaphase until all chromosomes are properly attached to spindle microtubules. Mps1 has emerged as a potential target for cancer therapy, and a variety of compounds have been developed to inhibit its kinase activity. Mutations in the catalytic domain of Mps1 that give rise to inhibitor resistance, but retain catalytic activity and do not display cross-resistance to other Mps1 inhibitors, have been described. Here we characterize the interactions of two such mutants, Mps1 C604Y and C604W, which raise resistance to two closely related compounds, NMS-P715 and its derivative Cpd-5, but not to the well characterized Mps1 inhibitor, reversine. We show that estimates of the IC 50 (employing a novel specific and efficient assay that utilizes a fluorescently labeled substrate) and the binding affinity ( K D ) indicate that, in both mutants, Cpd-5 should be better tolerated than the closely related NMS-P715. To gain further insight, we determined the crystal structure of the Mps1 kinase mutants bound to Cpd-5 and NMS-P715 and compared the binding modes of Cpd-5, NMS-P715, and reversine. The difference in steric hindrance between Tyr/Trp 604 and the trifluoromethoxy moiety of NMS-P715, the methoxy moiety of Cpd-5, and complete absence of such a group in reversine, account for differences we observe in vitro Our analysis enforces the notion that inhibitors targeting Mps1 drug-resistant mutations can emerge as a feasible intervention strategy based on existing scaffolds, if the clinical need arises.
- From the Divisions of Biochemistry and.
Organizational Affiliation: