Stable human IgG antibody therapeutics with native framework structure
Christie, M., Rouet, R., Nevoltris, D., Langley, D., Schofield, P., Fabri, L., Tingly, K., Lowe, D., Jermutus, L., Christ, D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Receptor tyrosine-protein kinase erbB-2 | A [auth C] | 658 | Homo sapiens | Mutation(s): 0 Gene Names: ERBB2, HER2, MLN19, NEU, NGL EC: 2.7.10.1 | 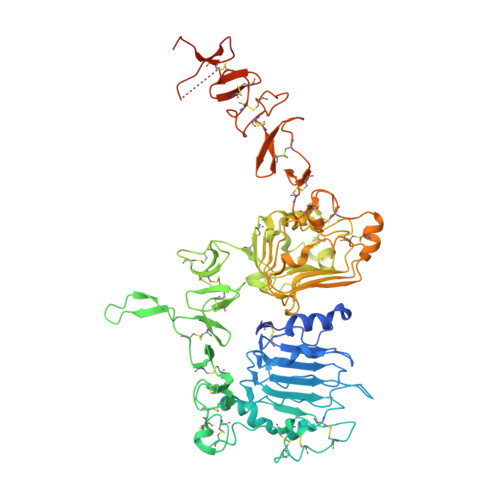 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P04626 (Homo sapiens) Explore P04626 Go to UniProtKB: P04626 | |||||
PHAROS: P04626 GTEx: ENSG00000141736 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P04626 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: P04626-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Herceptin light chain mutant | B [auth A] | 214 | Homo sapiens | Mutation(s): 0 | 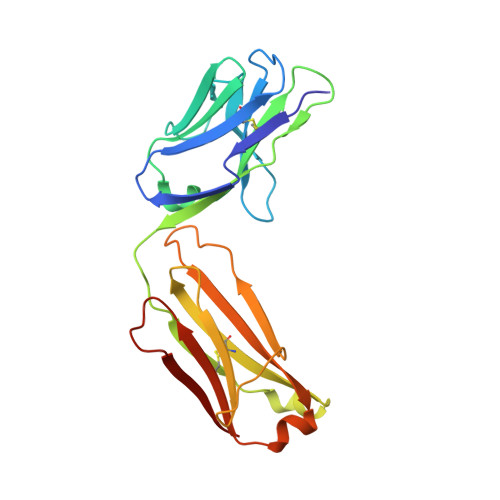 |
UniProt | |||||
Find proteins for Q7Z3Y4 (Homo sapiens) Explore Q7Z3Y4 Go to UniProtKB: Q7Z3Y4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7Z3Y4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Herceptin heavy chain Fab fragment mutant | C [auth B] | 231 | Homo sapiens | Mutation(s): 0 | 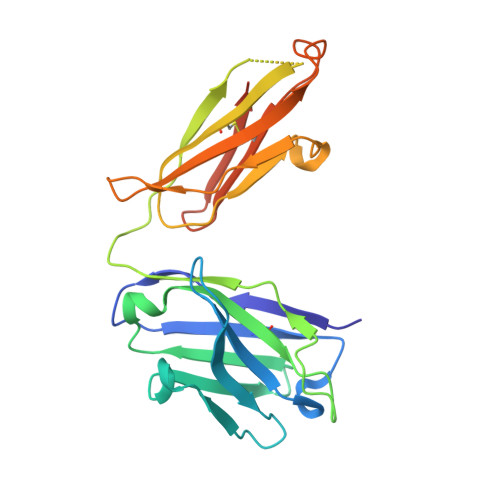 |
UniProt | |||||
Find proteins for P0DOX5 (Homo sapiens) Explore P0DOX5 Go to UniProtKB: P0DOX5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0DOX5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | D [auth C] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 62.86 | α = 90 |
| b = 114.46 | β = 90 |
| c = 204.279 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| MOSFLM | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Australian Research Council (ARC) | Australia | DE160100608 |
| Australian Research Council (ARC) | Australia | DP160104915 |