Structural Evidence for Rifampicin Monooxygenase Inactivating Rifampicin by Cleaving Its Ansa-Bridge.
Liu, L.K., Dai, Y., Abdelwahab, H., Sobrado, P., Tanner, J.J.(2018) Biochemistry 57: 2065-2068
- PubMed: 29578336
- DOI: https://doi.org/10.1021/acs.biochem.8b00190
- Primary Citation of Related Structures:
6C7S - PubMed Abstract:
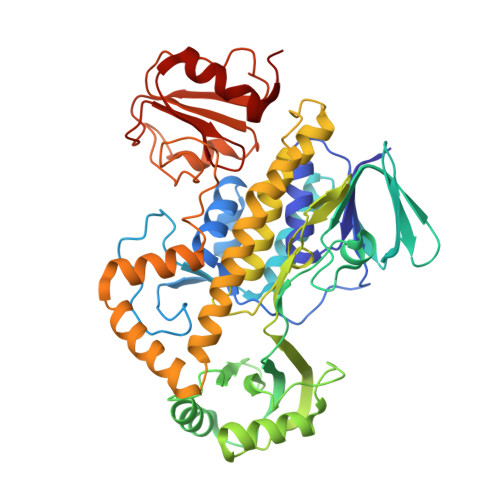
Rifampicin monooxygenase (RIFMO) decreases the potency of rifampicin (RIF) by converting it to oxidative products. Further decomposition of RIF has been observed in bacteria producing RIFMO and contributes to RIFMO-mediated drug resistance. Here we report the first crystal structure of RIFMO in complex with the hydroxylated RIF product. The 2.10 Å resolution structure reveals a breach of the ansa aliphatic chain of RIF between naphthoquinone C2 and amide N1. Our data suggest that RIFMO catalyzes the hydroxylation of RIF at the C2 atom followed by cleavage of the ansa linkage, which leads to inactivation of the antibiotic by preventing key contacts with the RNA polymerase target.
Organizational Affiliation:
Department of Biochemistry , Virginia Tech , Blacksburg , Virginia 24061 , United States.