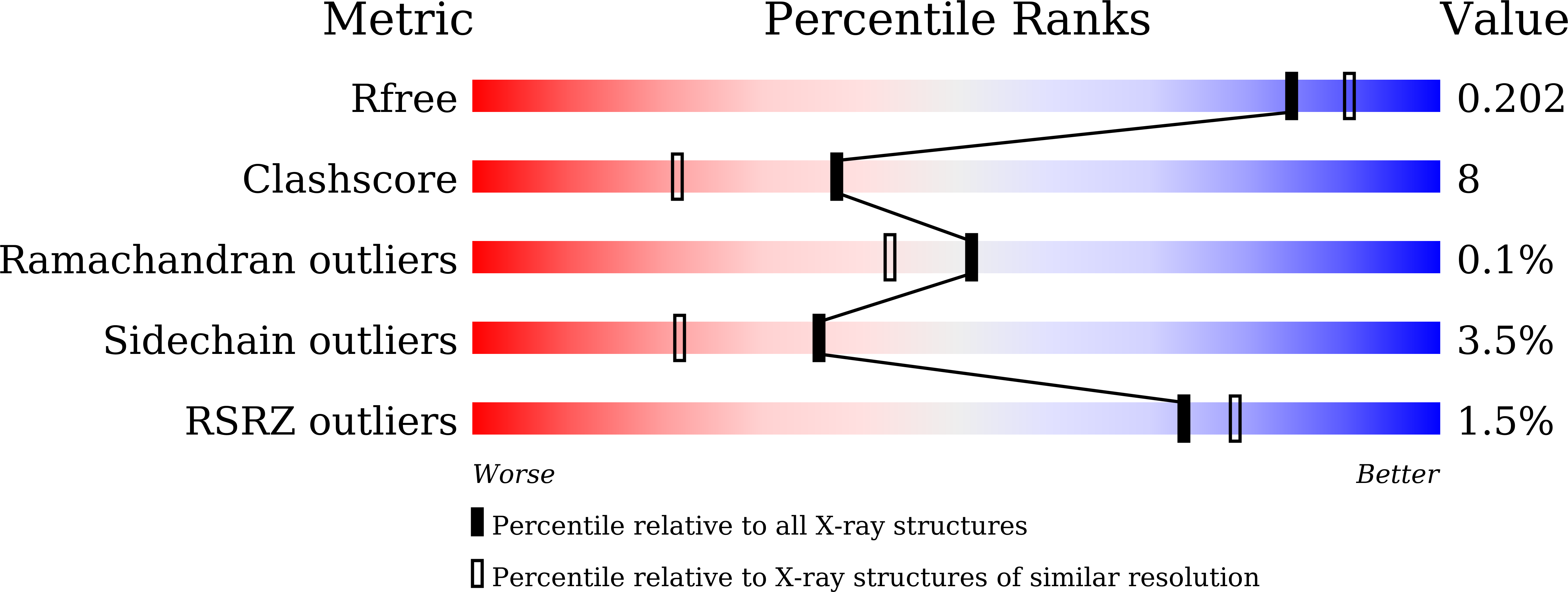
Characterization of dye-decolorizing peroxidase from Bacillus subtilis.
Dhankhar, P., Dalal, V., Mahto, J.K., Gurjar, B.R., Tomar, S., Sharma, A.K., Kumar, P.(2020) Arch Biochem Biophys 693: 108590-108590
- PubMed: 32971035
- DOI: https://doi.org/10.1016/j.abb.2020.108590
- Primary Citation of Related Structures:
6KMM, 6KMN - PubMed Abstract:
The dye-decolorizing peroxidases (DyPs) belong to a unique heme peroxidase family for their biotechnological potential to detoxify synthetic dyes. In this work, we have biochemically and structurally characterized the dye-decolorizing peroxidase from Bacillus subtilis (BsDyP). The biochemical studies of BsDyP demonstrate that pH 4.0 is optimum for the oxidation of malachite green (MG) and methyl violet (MV). However, it oxidizes the MG with higher catalytic efficiency (k cat /K m = 6.3 × 10 2 M -1 s -1 ), than MV (k cat /K m = 5.0 × 10 2 M -1 s -1 ). While reactive black 5 (RB5) is oxidized at pH 3.0 with the catalytic efficiency of k cat /K m = 3.6 × 10 2 M -1 s -1 . The calculated thermodynamic parameters by isothermal titration calorimetry (ITC) reveal the feasibility and spontaneity of dyes binding with BsDyP. Further, the crystal structures of a HEPES bound and unbound of BsDyP provide insight into the probable binding sites of the substrates. In BsDyP-HEPES bound structure, the HEPES-1 molecule is found in the heme cavity at the γ-edge, and another HEPES-2 molecule is bound ~16 Å away from the heme that is fenced by Ile231, Arg234, Ser235, Asp239, Glu334, and surface-exposed Tyr335 residues. Furthermore, the molecular docking, simulation, and MMPBSA studies support the binding of dyes at both the sites of BsDyP and produce lower-energy stable BsDyP-dyes complexes. Here, the BsDyP study allows the identification of its two potential binding sites and shows the oxidation of a variety of dyes. Structural and functional insight of BsDyP will facilitate its engineering for the improved decolorization of dyes.
Organizational Affiliation:
Department of Biotechnology, Indian Institute of Technology Roorkee, 247667, India.