Crystal Structure of the Ergothioneine Sulfoxide Synthase fromCandidatus Chloracidobacterium thermophilumand Structure-Guided Engineering To Modulate Its Substrate Selectivity.
Naowarojna, N., Irani, S., Hu, W., Cheng, R., Zhang, L., Li, X., Chen, J., Zhang, Y.J., Liu, P.(2019) ACS Catal 9: 6955-6961
- PubMed: 32257583
- DOI: https://doi.org/10.1021/acscatal.9b02054
- Primary Citation of Related Structures:
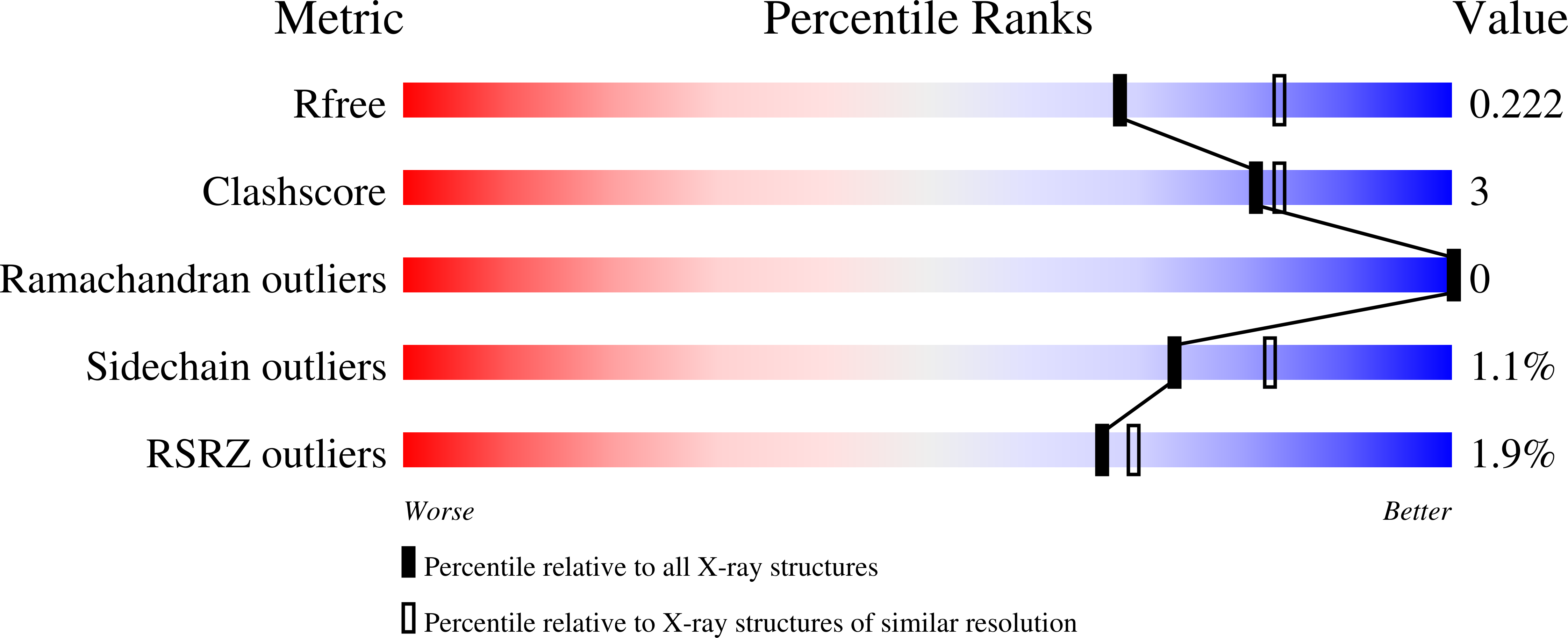
6O6L, 6O6M - PubMed Abstract:
Ergothioneine is a thiohistidine derivative with potential benefits on many aging-related diseases. The central step of aerobic ergothioneine biosynthesis is the oxidative C-S bond formation reaction catalyzed by mononuclear nonheme iron sulfoxide synthases (EgtB and Egt1). Thus far, only the Mycobacterium thermoresistibile EgtB (EgtB Mth ) crystal structure is available, while the structural information for the more industrially attractive Egt1 enzyme is not. Herein, we reported the crystal structure of the ergothioneine sulfoxide synthase (EgtB Cth ) from Candidatus Chloracidobacterium thermophilum. EgtB Cth has both EgtB- and Egt1-type of activities. Guided by the structural information, we conducted Rosetta Enzyme Design calculations, and we biochemically demonstrated that EgtB Cth can be engineered more toward Egt1-type of activity. This study provides information regarding the factors governing the substrate selectivity in Egt1- and EgtB-catalysis and lays the groundwork for future sulfoxide synthase engineering toward the development of an effective ergothioneine process through a synthetic biology approach.
Organizational Affiliation:
Department of Chemistry, Boston University, 590 Commonwealth Avenue, Boston, Massachusetts 02215, United States.