Crystal structure of GluN1/GluN2A NMDA receptor agonist binding domains with glycine and antagonist, 3-ethylphenyl-ACEPC
Syrenne, J.T., Mou, T.C., Tamborini, L., Pinto, A., Sprang, S.R., Hansen, K.B.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glutamate receptor ionotropic, NMDA 1 | 292 | Rattus norvegicus | Mutation(s): 0 Gene Names: Grin1, Nmdar1 | 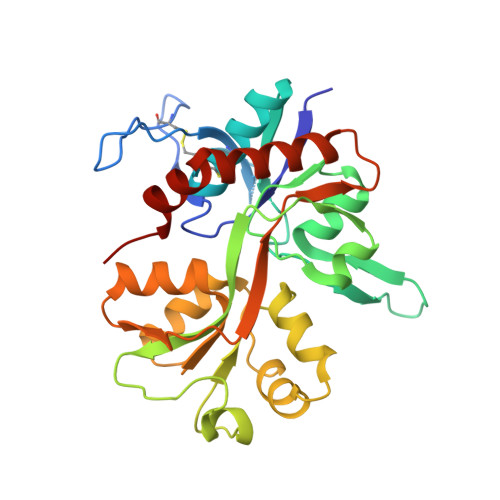 | |
UniProt | |||||
Find proteins for P35439 (Rattus norvegicus) Explore P35439 Go to UniProtKB: P35439 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P35439 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glutamate receptor ionotropic, NMDA 2A | 282 | Rattus norvegicus | Mutation(s): 0 Gene Names: Grin2a | 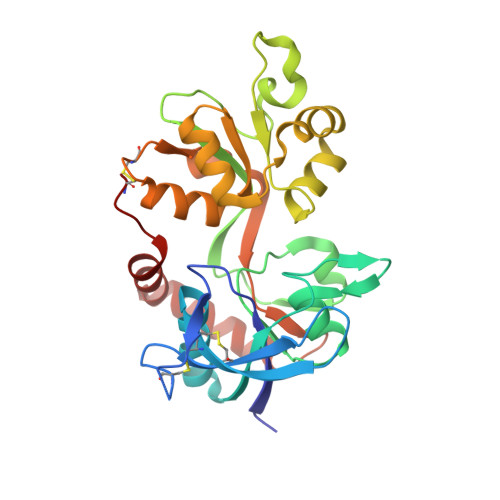 | |
UniProt | |||||
Find proteins for Q00959 (Rattus norvegicus) Explore Q00959 Go to UniProtKB: Q00959 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q00959 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| N9D (Subject of Investigation/LOI) Query on N9D | D [auth B] | (3R,5S)-5-[(2R)-2-amino-2-carboxyethyl]-1-(4-propylphenyl)pyrazolidine-3-carboxylic acid C16 H23 N3 O4 UPNZKJBKYMIUDF-BFHYXJOUSA-N |  | ||
| GLY (Subject of Investigation/LOI) Query on GLY | C [auth A] | GLYCINE C2 H5 N O2 DHMQDGOQFOQNFH-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 54.694 | α = 90 |
| b = 86.987 | β = 90 |
| c = 121.984 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | P20GM103546 |
| National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) | United States | R01NS097536 |