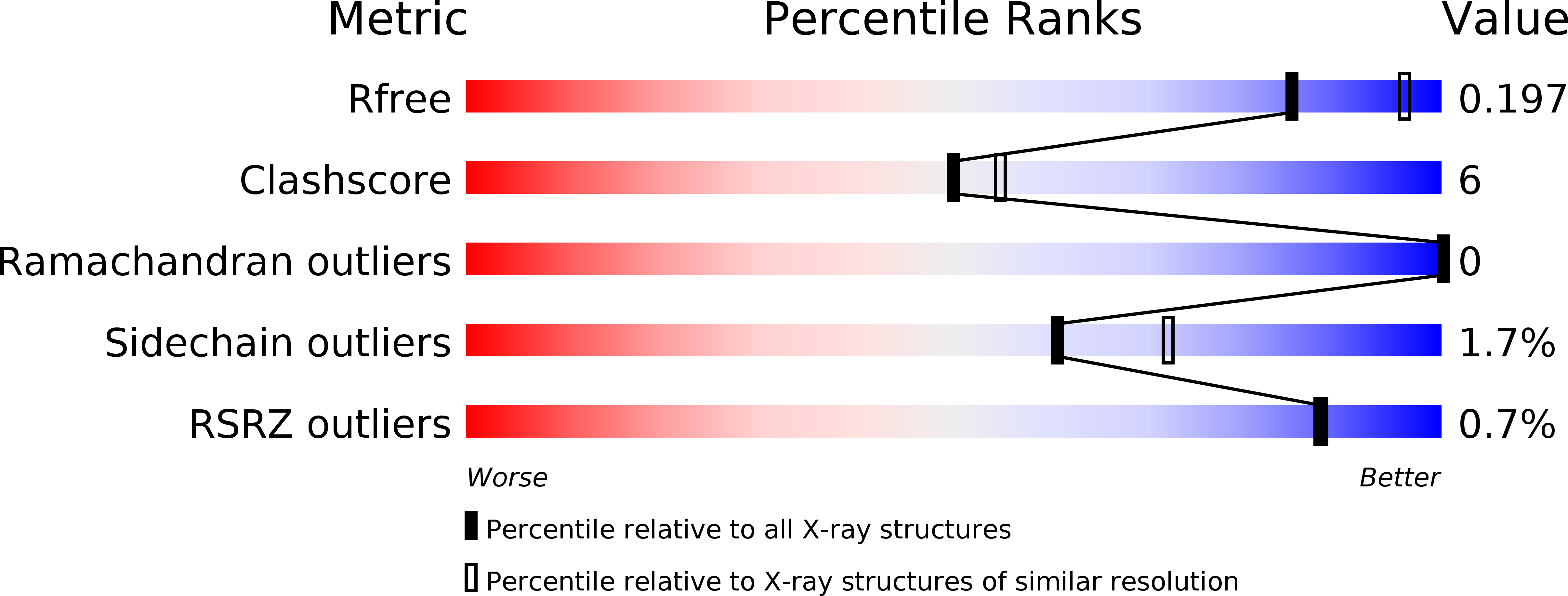
Crystal structure of dimericSynechococcusspermidine synthase with bound polyamine substrate and product.
Guedez, G., Pothipongsa, A., Siren, S., Liljeblad, A., Jantaro, S., Incharoensakdi, A., Salminen, T.A.(2019) Biochem J 476: 1009-1020
- PubMed: 30877192
- DOI: https://doi.org/10.1042/BCJ20180811
- Primary Citation of Related Structures:
6QMM - PubMed Abstract:
Spermidine is a ubiquitous polyamine synthesized by spermidine synthase (SPDS) from the substrates, putrescine and decarboxylated S-adenosylmethionine (dcAdoMet). SPDS is generally active as homodimer, but higher oligomerization states have been reported in SPDS from thermophiles, which are less specific to putrescine as the aminoacceptor substrate. Several crystal structures of SPDS have been solved with and without bound substrates and/or products as well as inhibitors. Here, we determined the crystal structure of SPDS from the cyanobacterium Synechococcus ( Sy SPDS) that is a homodimer, which we also observed in solution. Unlike crystal structures reported for bacterial and eukaryotic SPDS with bound ligands, Sy SPDS structure has not only bound putrescine substrate taken from the expression host, but also spermidine product most probably as a result of an enzymatic reaction. Hence, to the best of our knowledge, this is the first structure reported with both amino ligands in the same structure. Interestingly, the gate-keeping loop is disordered in the putrescine-bound monomer while it is stabilized in the spermidine-bound monomer of the Sy SPDS dimer. This confirms the gate-keeping loop as the key structural element that prepares the active site upon binding of dcAdoMet for the catalytic reaction of the amine donor and putrescine.
Organizational Affiliation:
Structural Bioinformatics Laboratory, Biochemistry, Faculty of Science and Engineering, Åbo Akademi University, Tykistökatu 6A, 20520 Turku, Finland.