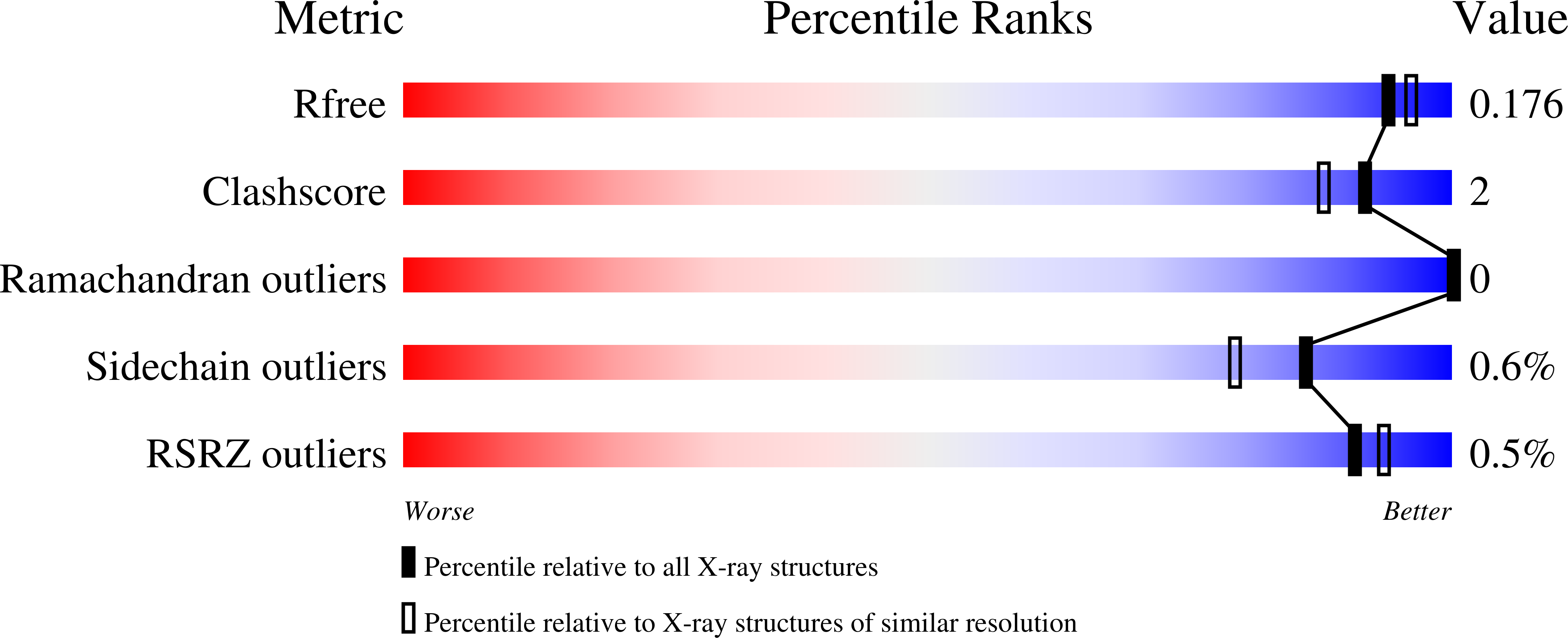
Probing the determinants of porosity in protein frameworks: co-crystals of cytochrome c and an octa-anionic calix[4]arene.
Alex, J.M., Brancatelli, G., Volpi, S., Bonaccorso, C., Casnati, A., Geremia, S., Crowley, P.B.(2020) Org Biomol Chem 18: 211-214
- PubMed: 31808772
- DOI: https://doi.org/10.1039/c9ob02275a
- Primary Citation of Related Structures:
6SUY - PubMed Abstract:
Sulfonato-calix[n]arenes (sclxn) are promising tools to generate crystalline protein frameworks. We report, for the first time, a lower rim functionalised octa-anionic calix[4]arene (sclx4mc) in complex with proteins. Two crystal structures of sclx4mc bound to yeast or horse heart cytochrome c (cytc) are described. Highly porous honeycomb or tubular assemblies were obtained with yeast or horse cytc, respectively. Related frameworks were obtained previously with sclx8 and sclx6 but not with sclx4, suggesting that the ligand charge is a determining factor.
Organizational Affiliation:
School of Chemistry, National University of Ireland Galway, University Road, Galway, H91 TK33, Ireland. peter.crowley@nuigalway.ie.