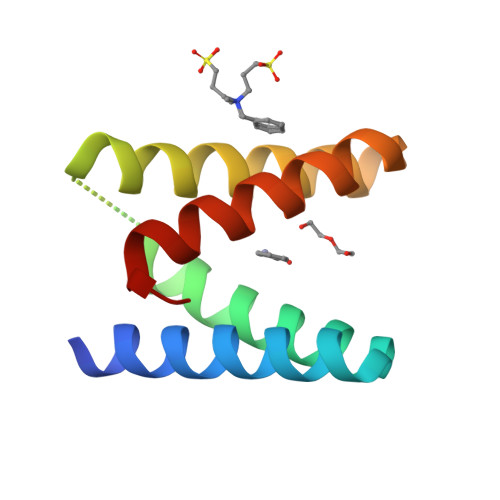
Efficient Lewis acid catalysis of an abiological reaction in a de novo protein scaffold.
Basler, S., Studer, S., Zou, Y., Mori, T., Ota, Y., Camus, A., Bunzel, H.A., Helgeson, R.C., Houk, K.N., Jimenez-Oses, G., Hilvert, D.(2021) Nat Chem 13: 231-235
- PubMed: 33526894
- DOI: https://doi.org/10.1038/s41557-020-00628-4
- Primary Citation of Related Structures:
6YPI, 7BWW - PubMed Abstract:
New enzyme catalysts are usually engineered by repurposing the active sites of natural proteins. Here we show that design and directed evolution can be used to transform a non-natural, functionally naive zinc-binding protein into a highly active catalyst for an abiological hetero-Diels-Alder reaction. The artificial metalloenzyme achieves >10 4 turnovers per active site, exerts absolute control over reaction pathway and product stereochemistry, and displays a catalytic proficiency (1/K TS = 2.9 × 10 10 M -1 ) that exceeds all previously characterized Diels-Alderases. These properties capitalize on effective Lewis acid catalysis, a chemical strategy for accelerating Diels-Alder reactions common in the laboratory but so far unknown in nature. Extension of this approach to other metal ions and other de novo scaffolds may propel the design field in exciting new directions.
Organizational Affiliation:
Laboratory of Organic Chemistry, ETH Zürich, Zürich, Switzerland.