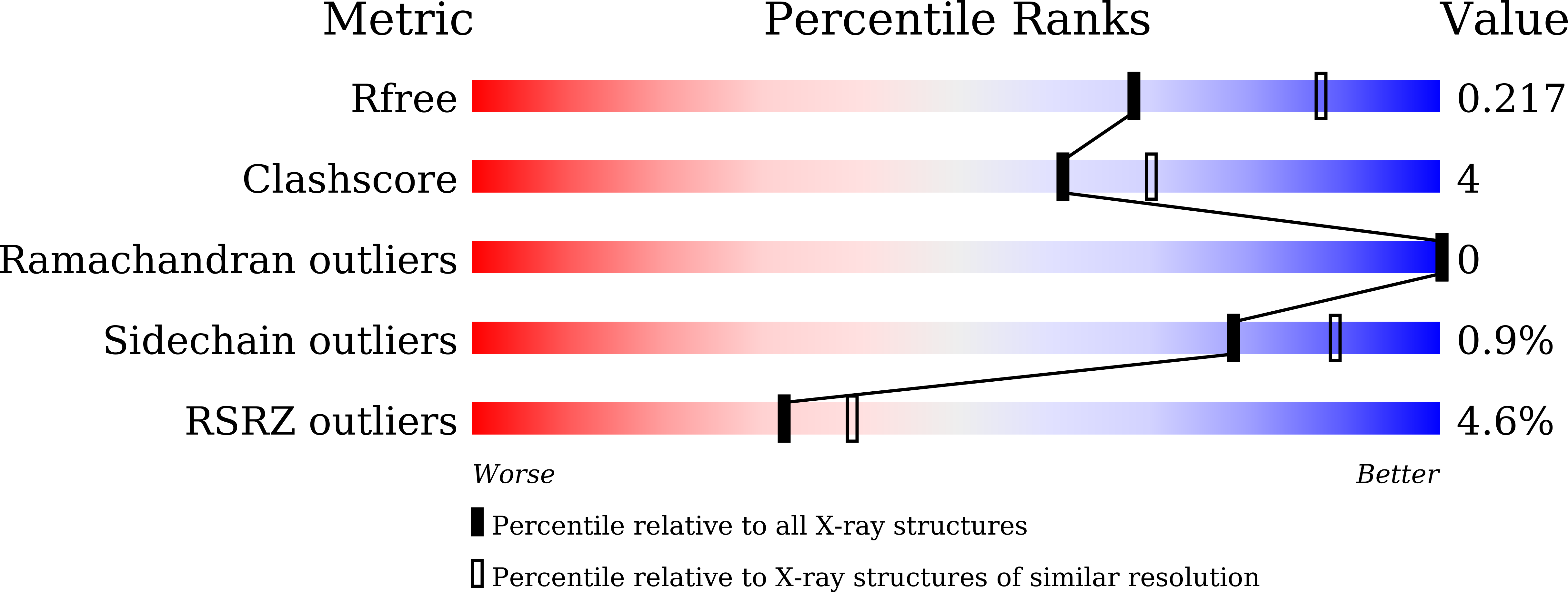
Cycloserine enantiomers inhibit PLP-dependent cysteine desulfurase SufS via distinct mechanisms.
Nakamura, R., Ogawa, S., Takahashi, Y., Fujishiro, T.(2022) FEBS J
- PubMed: 35395703
- DOI: https://doi.org/10.1111/febs.16455
- Primary Citation of Related Structures:
6KFY, 7CEO, 7CEP, 7CEQ, 7CER, 7CES, 7CET, 7CEU, 7E6A, 7E6B, 7E6C, 7E6D, 7E6E, 7E6F - PubMed Abstract:
The cysteine desulfurase SufS is a pyridoxal-5'-phosphate-dependent enzyme and is essential for the SUF system, which participates in iron-sulfur cluster biosynthesis. Inhibition of SufS in the SUF system by D-cycloserine (DCS) in Plasmodium falciparum apicoplast has recently been reported, indicating that SufS could be a target for malaria therapeutics. However, the mechanistic details underlying the inhibition of SufS by DCS have not yet been clarified. Moreover, inhibition of SufS by the other enantiomer, L-cycloserine (LCS), has not been investigated. Herein, we investigated the structure-based inhibition mechanisms of SufS by DCS and LCS using Bacillus subtilis SufS, whose catalytic mechanism has been well characterized in comparison to that of the P. falciparum SufS. Surprisingly, DCS- and LCS-mediated inhibitions of SufS occur via distinct mechanisms resulting in pyridoxamine-5'-phosphate (PMP) in DCS-mediated inhibition and PMP-3-hydroxyisoxazole adduct (PMP-isoxazole) in LCS-mediated inhibition. Biochemical and structural evaluation of SufS variants identified conserved His and Arg residues at the active site as the key determinants of the distinct inhibition mechanisms. The importance of structural elements involved in DCS and LCS-mediated inhibitions of SufS provides valuable insights for the structure-based design of new drugs targeting SufS. DATABASE: Structural data are available in PDB database under the accession numbers 6KFY, 7CEO, 7CEP, 7CEQ, 7CER, 7CES, 7CET, 7CEU, 7E6A, 7E6B, 7E6C, 7E6D, 7E6E, and 7E6F.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Graduate School of Science and Engineering, Saitama University, Japan.