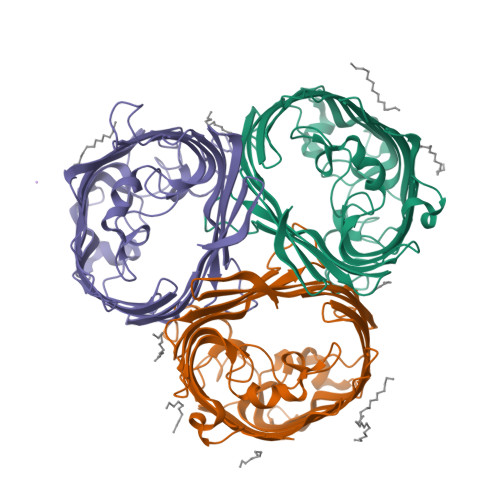
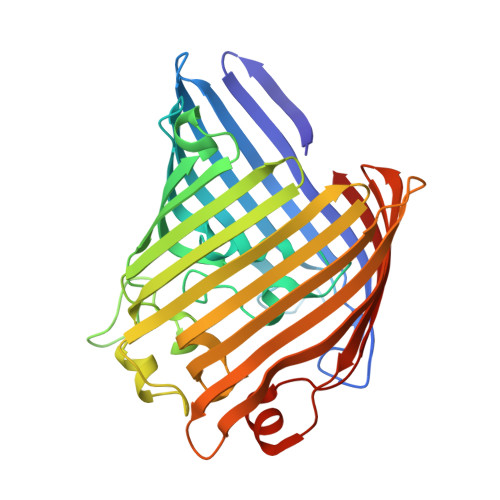
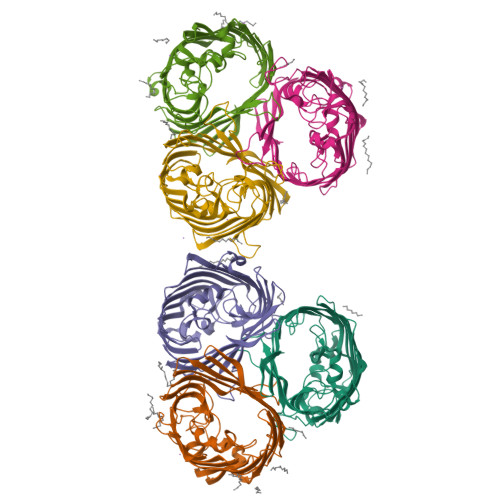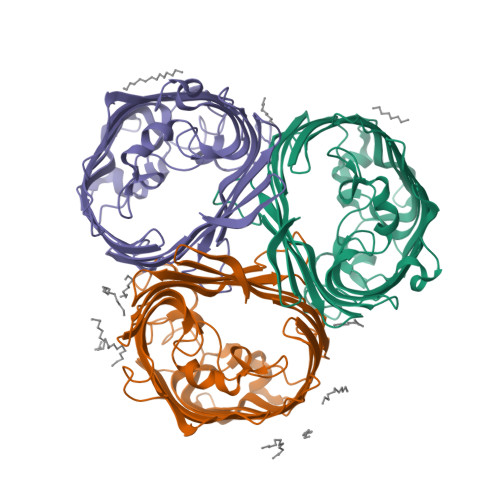Widespread emergence of OmpK36 loop 3 insertions among multidrug-resistant clones of Klebsiella pneumoniae.
David, S., Wong, J.L.C., Sanchez-Garrido, J., Kwong, H.S., Low, W.W., Morecchiato, F., Giani, T., Rossolini, G.M., Brett, S.J., Clements, A., Beis, K., Aanensen, D.M., Frankel, G.(2022) PLoS Pathog 18: e1010334-e1010334
- PubMed: 35816554
- DOI: https://doi.org/10.1371/journal.ppat.1010334
- Primary Citation of Related Structures:
7PZF, 7Q3T - PubMed Abstract:
Mutations in outer membrane porins act in synergy with carbapenemase enzymes to increase carbapenem resistance in the important nosocomial pathogen, Klebsiella pneumoniae (KP). A key example is a di-amino acid insertion, Glycine-Aspartate (GD), in the extracellular loop 3 (L3) region of OmpK36 which constricts the pore and restricts entry of carbapenems into the bacterial cell. Here we combined genomic and experimental approaches to characterise the diversity, spread and impact of different L3 insertion types in OmpK36. We identified L3 insertions in 3588 (24.1%) of 14,888 KP genomes with an intact ompK36 gene from a global collection. GD insertions were most common, with a high concentration in the ST258/512 clone that has spread widely in Europe and the Americas. Aspartate (D) and Threonine-Aspartate (TD) insertions were prevalent in genomes from Asia, due in part to acquisitions by KP sequence types ST16 and ST231 and subsequent clonal expansions. By solving the crystal structures of novel OmpK36 variants, we found that the TD insertion causes a pore constriction of 41%, significantly greater than that achieved by GD (10%) or D (8%), resulting in the highest levels of resistance to selected antibiotics. We show that in the absence of antibiotics KP mutants harbouring these L3 insertions exhibit both an in vitro and in vivo competitive disadvantage relative to the isogenic parental strain expressing wild type OmpK36. We propose that this explains the reversion of GD and TD insertions observed at low frequency among KP genomes. Finally, we demonstrate that strains expressing L3 insertions remain susceptible to drugs targeting carbapenemase-producing KP, including novel beta lactam-beta lactamase inhibitor combinations. This study provides a contemporary global view of OmpK36-mediated resistance mechanisms in KP, integrating surveillance and experimental data to guide treatment and drug development strategies.
Organizational Affiliation:
Centre for Genomic Pathogen Surveillance, Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, University of Oxford, Oxford, United Kingdom.