Functional and Structural Insights into Human PPAR alpha / delta / gamma Subtype Selectivity of Bezafibrate, Fenofibric Acid, and Pemafibrate.
Honda, A., Kamata, S., Akahane, M., Machida, Y., Uchii, K., Shiiyama, Y., Habu, Y., Miyawaki, S., Kaneko, C., Oyama, T., Ishii, I.(2022) Int J Mol Sci 23
- PubMed: 35563117
- DOI: https://doi.org/10.3390/ijms23094726
- Primary Citation of Related Structures:
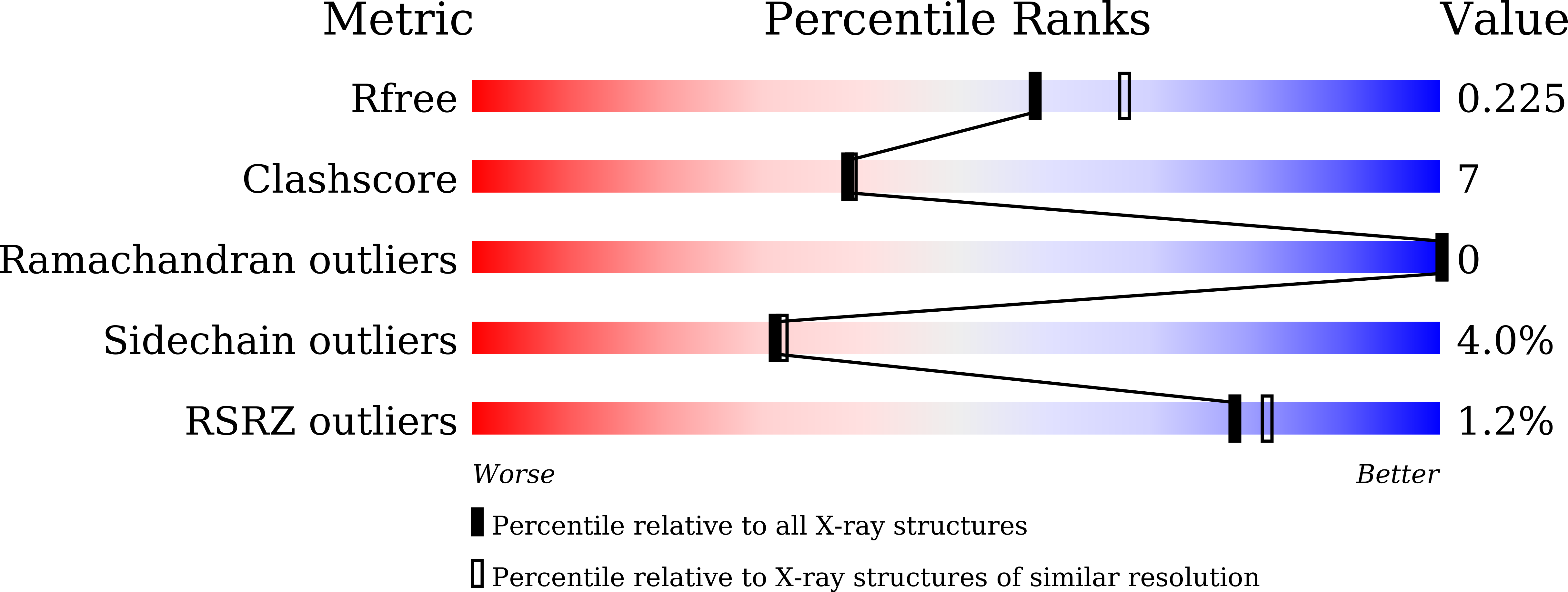
7WGL, 7WGN, 7WGO, 7WGP, 7WGQ - PubMed Abstract:
Among the agonists against three peroxisome proliferator-activated receptor (PPAR) subtypes, those against PPARα (fibrates) and PPARγ (glitazones) are currently used to treat dyslipidemia and type 2 diabetes, respectively, whereas PPARδ agonists are expected to be the next-generation metabolic disease drug. In addition, some dual/pan PPAR agonists are currently being investigated via clinical trials as one of the first curative drugs against nonalcoholic fatty liver disease (NAFLD). Because PPARα/δ/γ share considerable amino acid identity and three-dimensional structures, especially in ligand-binding domains (LBDs), clinically approved fibrates, such as bezafibrate, fenofibric acid, and pemafibrate, could also act on PPARδ/γ when used as anti-NAFLD drugs. Therefore, this study examined their PPARα/δ/γ selectivity using three independent assays-a dual luciferase-based GAL4 transactivation assay for COS-7 cells, time-resolved fluorescence resonance energy transfer-based coactivator recruitment assay, and circular dichroism spectroscopy-based thermostability assay. Although the efficacy and efficiency highly varied between agonists, assay types, and PPAR subtypes, the three fibrates, except fenofibric acid that did not affect PPARδ-mediated transactivation and coactivator recruitment, activated all PPAR subtypes in those assays. Furthermore, we aimed to obtain cocrystal structures of PPARδ/γ-LBD and the three fibrates via X-ray diffraction and versatile crystallization methods, which we recently used to obtain 34 structures of PPARα-LBD cocrystallized with 17 ligands, including the fibrates. We herein reveal five novel high-resolution structures of PPARδ/γ-bezafibrate, PPARγ-fenofibric acid, and PPARδ/γ-pemafibrate, thereby providing the molecular basis for their application beyond dyslipidemia treatment.
Organizational Affiliation:
Department of Health Chemistry, Showa Pharmaceutical University, Machida 194-8543, Tokyo, Japan.