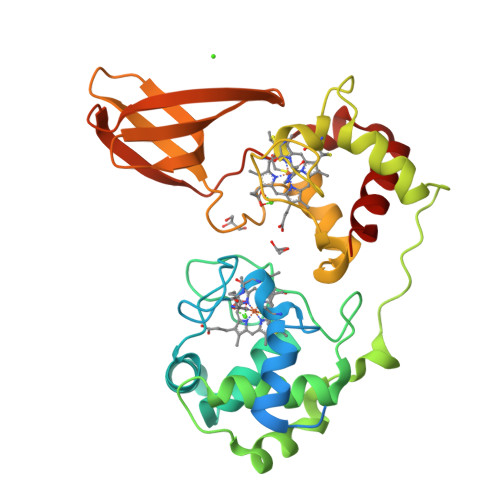
Redox potential tuning by calcium ions in a novel c-type cytochrome from an anammox organism.
Akram, M., Hauser, D., Dietl, A., Steigleder, M., Ullmann, G.M., Barends, T.R.M.(2024) J Biological Chem : 108082-108082
- PubMed: 39675707
- DOI: https://doi.org/10.1016/j.jbc.2024.108082
- Primary Citation of Related Structures:
7ZS0, 7ZS1, 7ZS2, 9FBK - PubMed Abstract:
The electrochemical potentials of redox-active proteins need to be tuned accurately to the correct values for proper biological function. Here, we describe a diheme cytochrome c with high heme redox potentials of about +350 mV, despite having a large overall negative charge, which typically reduces redox potentials. High-resolution crystal structures, spectroelectrochemical measurements, and high-end computational methods show how this is achieved: each heme iron has a calcium cation positioned next to it at a distance of only 6.9 Å, raising their redox potentials by several hundred millivolts through electrostatic interaction. We suggest that this has evolved to provide the protein with a high redox potential despite its large negative surface charge, which it likely requires for interactions with redox partners.
Organizational Affiliation:
Department of Biomolecular Mechanisms, Max Planck Institute for Medical Research, Heidelberg, Germany.