Redox potential tuning by calcium ions in a novel c-type cytochrome from an anammox organism
Akram, M., Barends, T.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Hypothetical (Diheme) protein | 316 | Candidatus Kuenenia | Mutation(s): 1 Gene Names: KsCSTR_07890, KSMBR1_1466, kustd1711 | 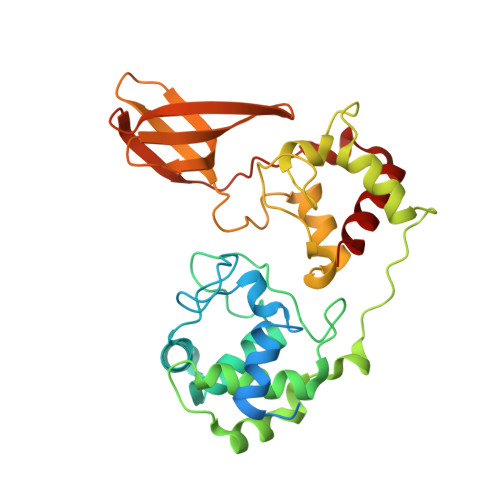 | |
UniProt | |||||
Find proteins for Q1PZE6 (Kuenenia stuttgartiensis) Explore Q1PZE6 Go to UniProtKB: Q1PZE6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q1PZE6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HEC (Subject of Investigation/LOI) Query on HEC | F [auth A], G [auth A] | HEME C C34 H34 Fe N4 O4 HXQIYSLZKNYNMH-LJNAALQVSA-N |  | ||
| IMD Query on IMD | H [auth A] | IMIDAZOLE C3 H5 N2 RAXXELZNTBOGNW-UHFFFAOYSA-O |  | ||
| CA (Subject of Investigation/LOI) Query on CA | B [auth A], C [auth A], D [auth A], E [auth A] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 62.65 | α = 90 |
| b = 92.71 | β = 90 |
| c = 55.23 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Max Planck Society | Germany | -- |