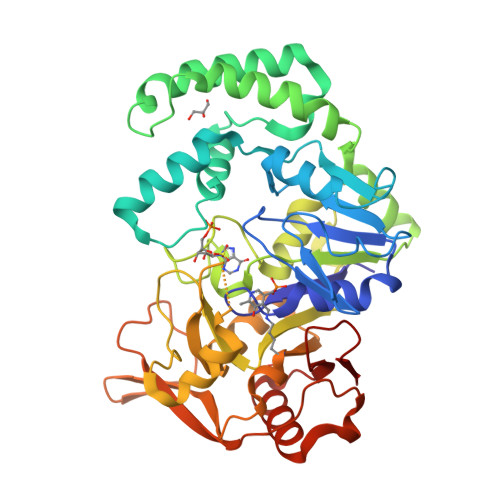
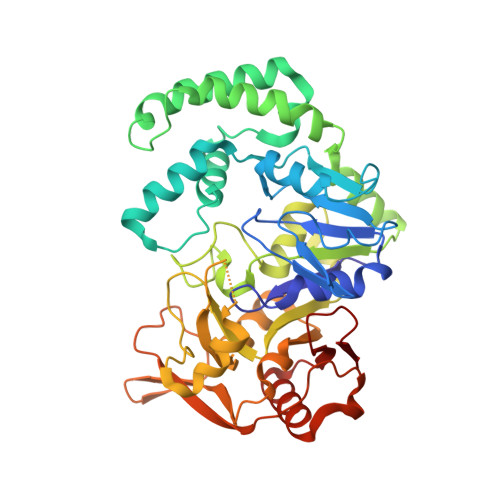
Vitamin B6 inhibits activity of Helicobacter pylori adenylosuccinate synthetase and growth of reference and clinical, antibiotic-resistant H. pylori strains.
Wojtys, M.I., Maksymiuk, W., Narczyk, M., Bubic, A., Asler, I.L., Krzyzek, P., Gosciniak, G., Jagusztyn-Krynicka, E.K., Bzowska, A.(2024) J Enzyme Inhib Med Chem 39: 2372734-2372734
- PubMed: 39149761
- DOI: https://doi.org/10.1080/14756366.2024.2372734
- Primary Citation of Related Structures:
8QWA - PubMed Abstract:
The current therapies against gastric pathogen Helicobacter pylori are ineffective in over 20% of patients. Enzymes belonging to the purine salvage pathway are considered as novel drug targets in this pathogen. Therefore, the main aim of the current study was to determine the antibacterial activity of pyridoxal 5'-phosphate (PLP), an active form of vitamin B6, against reference and clinical strains of H. pylori . Using a broad set of microbiological, physicochemical (UV absorption, LC-MS, X-ray analysis) and in silico experiments, we were able to prove that PLP inhibits adenylosuccinate synthetase (AdSS) from H. pylori by the competition with GTP (IC 50 eq ∼30 nM). This behaviour was attributed to formation of a Schiff base with a lysine residue (a covalent bond with Lys322 in the GTP binding site of AdSS) and was potentiated by the presence of vitamin C. This antibacterial activity of PLP gives hope for its future use against H. pylori .
Organizational Affiliation:
Division of Biophysics, Institute of Experimental Physics, Faculty of Physics, University of Warsaw, Warsaw, Poland.