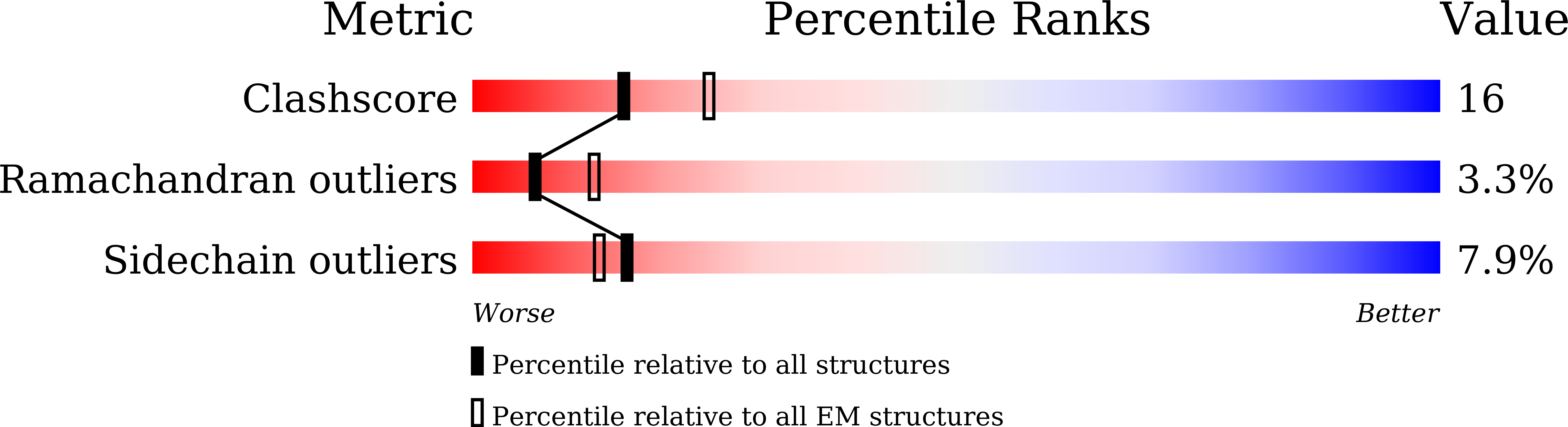Key mechanistic features of the trade-off between antibody escape and host cell binding in the SARS-CoV-2 Omicron variant spike proteins.
Li, W., Xu, Z., Niu, T., Xie, Y., Zhao, Z., Li, D., He, Q., Sun, W., Shi, K., Guo, W., Chang, Z., Liu, K., Fan, Z., Qi, J., Gao, G.F.(2024) EMBO J 43: 1484-1498
- PubMed: 38467833
- DOI: https://doi.org/10.1038/s44318-024-00062-z
- Primary Citation of Related Structures:
8WDR, 8WDS, 8WDY, 8WDZ, 8WE0, 8WE1, 8WE4 - PubMed Abstract:
Since SARS-CoV-2 Omicron variant emerged, it is constantly evolving into multiple sub-variants, including BF.7, BQ.1, BQ.1.1, XBB, XBB.1.5 and the recently emerged BA.2.86 and JN.1. Receptor binding and immune evasion are recognized as two major drivers for evolution of the receptor binding domain (RBD) of the SARS-CoV-2 spike (S) protein. However, the underlying mechanism of interplay between two factors remains incompletely understood. Herein, we determined the structures of human ACE2 complexed with BF.7, BQ.1, BQ.1.1, XBB and XBB.1.5 RBDs. Based on the ACE2/RBD structures of these sub-variants and a comparison with the known complex structures, we found that R346T substitution in the RBD enhanced ACE2 binding upon an interaction with the residue R493, but not Q493, via a mechanism involving long-range conformation changes. Furthermore, we found that R493Q and F486V exert a balanced impact, through which immune evasion capability was somewhat compromised to achieve an optimal receptor binding. We propose a "two-steps-forward and one-step-backward" model to describe such a compromise between receptor binding affinity and immune evasion during RBD evolution of Omicron sub-variants.
Organizational Affiliation:
CAS Key Laboratory of Pathogen Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences (CAS), Beijing, China.