A noncanonical glycoprotein H complex enhances cytomegalovirus entry.
Norris, M.J., Henderson, L.A., Siddiquey, M.N.A., Yin, J., Yoo, K., Brunel, S., Saphire, E.O., Benedict, C.A., Kamil, J.P.(2024) bioRxiv
- PubMed: 39416215
- DOI: https://doi.org/10.1101/2024.10.13.617647
- Primary Citation of Related Structures:
9DIX, 9DIY - PubMed Abstract:
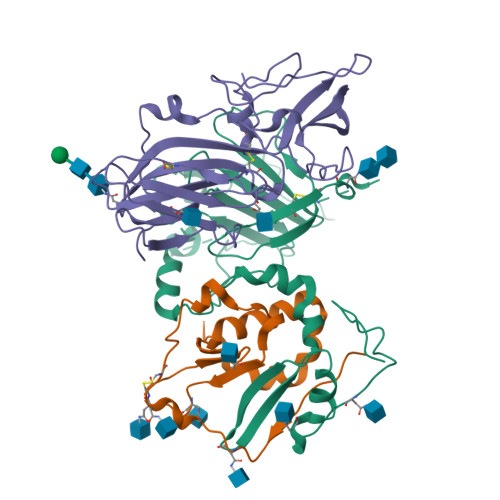
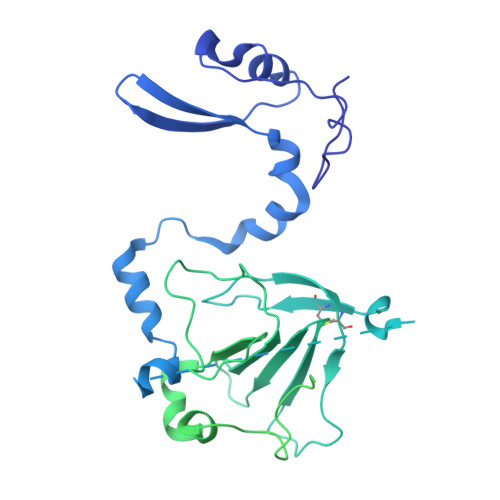
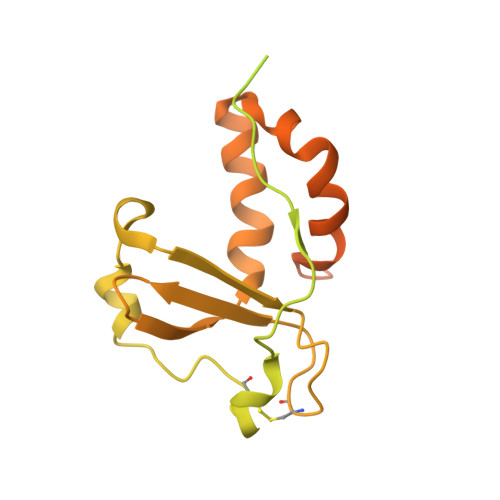
Human cytomegalovirus (HCMV) causes severe birth defects, lifelong health complications, and $4 billion in annual costs in the United States alone. A major challenge in vaccine design is the incomplete understanding of the diverse protein complexes the virus uses to infect cells. In Herpesviridae , the gH/gL glycoprotein heterodimer is expected to be a basal element of virion cell entry machinery. For HCMV, gH/gL forms a "trimer" with gO and a "pentamer" with UL128, UL130, and UL131A, with each complex binding distinct receptors to enter varied cell types. Here, we reveal a third glycoprotein complex, abundant in HCMV virions, which significantly enhances infection of endothelial cells. In this "3-mer" complex, gH, without gL, associates with UL116 and UL141, an immunoevasin previously known to function in an intracellular role. Cryo-EM reveals the virion-surface 3-mer is structurally unique among Herpesviridae gH complexes, with gH-only scaffolding, UL141-mediated dimerization and a heavily glycosylated UL116 cap. Given that antibodies directed at gH and UL141 each can restrict HCMV replication, our work highlights this virion surface complex as a new target for vaccines and antiviral therapies.
Organizational Affiliation:
Center for Vaccine Innovation, La Jolla Institute for Immunology, La Jolla, CA.