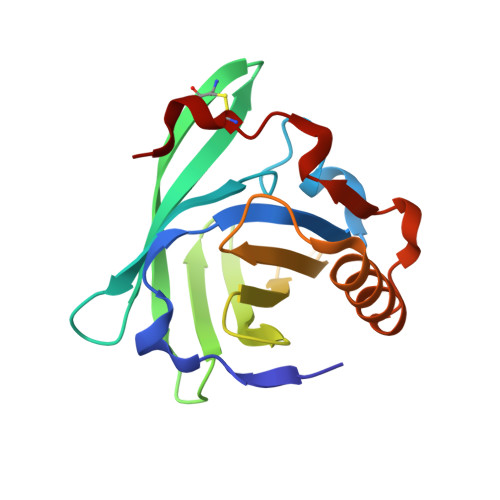
Crystal structure of the allergen Equ c 1. A dimeric lipocalin with restricted IgE-reactive epitopes.
Lascombe, M.B., Gregoire, C., Poncet, P., Tavares, G.A., Rosinski-Chupin, I., Rabillon, J., Goubran-Botros, H., Mazie, J.C., David, B., Alzari, P.M.(2000) J Biol Chem 275: 21572-21577
- PubMed: 10787420
- DOI: https://doi.org/10.1074/jbc.M002854200
- Primary Citation of Related Structures:
1EW3 - PubMed Abstract:
The three-dimensional structure of the major horse allergen Equ c 1 has been determined at 2.3 A resolution by x-ray crystallography. Equ c 1 displays the typical fold of lipocalins, a beta-barrel flanked by a C-terminal alpha-helix. The space between the two beta-sheets of the barrel defines an internal cavity that could serve, as in other lipocalins, for the binding and transport of small hydrophobic ligands. Equ c 1 crystallizes in a novel dimeric form, which is distinct from that observed in other lipocalin dimers and corresponds to the functional form of the allergen. Binding studies of point mutants of the allergen with specific monoclonal antibodies raised in mouse and IgE serum from horse allergic patients allowed to identify putative B cell antigenic determinants. In addition, total inhibition of IgE serum recognition by a single specific monoclonal antibody revealed the restricted nature of the IgE binding target on the molecular surface of Equ c 1.
Organizational Affiliation:
Unité de Biochimie Structurale (CNRS URA 2185), Unité d'Immuno-Allergie, 25 et 28 rue du Docteur Roux, 75724 Paris Cedex 15, France.